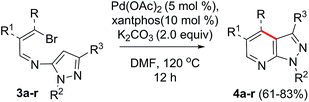Palladium mediated regioselective intramolecular Heck reaction: synthesis of 1,3,4-trisubstituted pyrazolo[3,4-b]pyridines, 3H-pyrazolo[3,4-c]isoquinolines and 3H-pyrazolo[4,3-f][1,7]naphthyridines†
Rashmi Prakash,
Kommuri Shekarrao,
Pallabi Saikia,
Sanjib Gogoi* and
Romesh C. Boruah*
Medicinal Chemistry Division, North-East Institute of Science and Technology, Jorhat, Assam 785 006, India. E-mail: skgogoi1@gmail.com; rc_boruah@yahoo.co.in; Fax: +91 3762370011; Tel: +91 3762372948
First published on 18th February 2015
Abstract
An efficient method was developed for the synthesis of 1,3,4-trisubstituted pyrazolo[3,4-b]pyridines, 3H-pyrazolo[3,4-c]isoquinolines and 3H-pyrazolo[4,3-f][1,7]naphthyridines via intramolecular Heck reactions of imine derivatives of β-halovinyl/aryl aldehydes and 5-aminopyrazoles. This palladium acetate catalyzed reaction afforded good yields of these pyrazolo fused compounds under thermal conditions in the presence of xantphos as the ligand.
Introduction
The pyrazolo[3,4-b]pyridine moieties are found to be important building blocks in both natural and synthetic bioactive compounds.1 They show a wide range of biological activities such as anxiolytic, inhibitors of xanthine oxidases, kinase inhibitors of p38a, potent and selective inhibitors of A1 adenosine receptors, glycogen synthase kinase-3 (GSK-3) inhibitors, inhibitors of B-RafV600E and cholesterol formation.2 Similarly, 3H-pyrazolo[3,4-c]isoquinoline and 3H-pyrazolo[4,3-f][1,7]naphthyridine derivatives are also known for their wide range of biological activities.3In view of the biological importance of these pyrazolo[3,4-b]pyridines, considerable attention has been focused on the synthesis of these fused heterocycles. There are various methods reported for the synthesis of pyrazolo[3,4-b]pyridines of types I–IV (Fig. 1), using typically multi-component reactions.4 Very recently, we reported the synthesis of pyrazolo[3,4-b]pyridines of type V (Fig. 1) by the reaction of β-bromovinyl aldehyde with 5-aminopyrazole in the presence of palladium catalyst via cascade amination/imination/cycloaddition reactions.5 In spite of various methods available for the construction of pyrazolo[3,4-b]pyridines with different substituent patterns, to the best of our knowledge, the synthesis of 1,3,4-trisubstituted pyrazolo[3,4-b]pyridines (VI, Fig. 1) is not investigated so far in the literature. Our reported methodology for pyrazolo[3,4-b]pyridines V,5 could not be applied for the synthesis of these pyrazolo[3,4-b]pyridines VI. Similarly, there are only few references known in the literature for the synthesis of 3H-pyrazolo[3,4-c]isoquinolines and 3H-pyrazolo[4,3-f][1,7]naphthyridine derivatives. The only metal catalyzed methodology for the synthesis of 3H-pyrazolo[3,4-c]isoquinolines was reported recently by Batra and co-workers using Pd–Cu catalyzed cascade imination/intramolecular decarboxylative coupling reaction, which required the presence of one acid group at δ-position of 5-amino pyrazoles (Scheme 1, eqn (1)).6 The methodology developed by Bogza and co-workers is limited to the syntheses of only 7,8-dimethoxy-3H-pyrazolo[3,4-c]isoquinolines, which was performed by the reflux reaction of 4-(3,4-dimethoxyphenyl)-5-aminopyrazoles with aromatic aldehydes in strong acidic media.3a,3d Recently, Jiang and co-workers reported the synthesis of pyrazolo-fused 1,7-naphthyridines by the domino reactions of arylglyoxals with pyrazol-5-amine.3b In spite of the importance of these pyrazolo fused heterocycles, a general route for the synthesis of these novel heterocycles that have wide substrate scope is so far absent in the literature. Thus, the development of new routes to construct these pyrazolo skeletons is highly essential.
Intramolecular Heck reactions have become a powerful method for the construction of fused heteroaromatic compounds as well as cyclic natural products.7 Palladium(II) catalyzed intramolecular Heck reactions have been using effectively by different research groups for the construction of different heterocycles such as isoquinolines, indolines, benzofurans etc.8 Herein, we wish to report the first attempt of intramolelar Heck reaction of imine derivatives of β-bromovinyl/aryl aldehydes and 5-aminopyrazoles for the construction of different important pyrazole fused heterocycles such as 1,3,4-trisubstituted pyrazolo[3,4-b]pyridines, 3H-pyrazolo[3,4-c]isoquinolines and 3H-pyrazolo[4,3-f][1,7]naphthyridines.
Results and discussion
At the beginning, our attempt to perform the PdCl2 catalyzed intramolecular coupling reaction of the in situ generated imine derivative of β-bromo-β-(2-napthyl)vinyl aldehyde (1a, 1.0 mmol) and 1-phenyl-3-methyl-5-aminopyrazole (2a, 1.0 mmol) in DMF, furnished only the previously reported pyrazolo[3,4-b]pyridine 5a5 (Scheme 2). Probably, the presence of water molecules helped the partial hydrolysis of the imines to generate the free amines, which in presence of the Pd catalyst affords compound 5a, via previously reported cascade imination/coupling/cycloaddition reaction.5 To overcome the formation of compound 5a, then we tried the intramolecular coupling reaction of pure imine derivative 3a (1.0 mmol), with PdCl2 (5.0 mol%), PPh3 (10.0 mol%) and K2CO3 (2.0 mmol) in DMF (Table 1). To our delight, after heating the reaction mixture for 12 hours at 120 °C, the reaction afforded pyrazolo[3,4-b]pyridine derivative 4a in 34% yield (entry 1, Table 1). This pyrazolo[3,4-b]pyridine derivative 4a was fully characterized by 1H NMR, 13C NMR and mass spectroscopy. Then, we studied some other palladium catalysts and ligands to determine the ideal reaction condition for the synthesis of compound 4a which are shown in Table 1. Moreover, we screened some bases and solvents which were frequently used to perform the intramolecular Heck reactions (Table 1).9 Screening of palladium catalysts such as Pd(OAc)2, PdCl2(PPh3)2 and Pd(TFA)2 revealed Pd(OAc)2 as the best catalyst to perform this reaction (entries 2–4). In absence of the catalyst, the reaction did not work (entry 5). In addition, screening of solvents disclosed DMF as the best solvent to perform this reaction (entries 2, 6 and 7). Further studies on bases revealed K2CO3 as the best base, although Cs2CO3 worked efficiently, the yield of 4a did not exceed 68% (entries 2, 8 and 9). For further improvement of the yield, we next focused our attention on the ligands. In absence of the ligand, the reaction gave inferior result, whereas, the use of the monophosphine ligand P(o-tol)3 provided the desired compound in 67% yield (entries 10 and 11). Then, we further tested some bisphosphine ligands such as dppf and xantphos for optimization of the reaction (entries 12 and 13).| Entry | Pd catalyst | Base | Ligand | Solvent | 4ab (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 3a (1.0 mmol), catalyst (5.0 mol%), base (2.0 equiv), ligand (10.0 mol%), solvent (3.0 mL), 120 °C, 12 h.b Isolated yield.c Using 2.5 mol% of catalyst.d Using 7.5 mol% of catalyst. | |||||
| 1 | PdCl2 | K2CO3 | PPh3 | DMF | 34 |
| 2 | Pd(OAc)2 | K2CO3 | PPh3 | DMF | 70 |
| 3 | PdCl2(PPh3)2 | K2CO3 | PPh3 | DMF | 49 |
| 4 | Pd(TFA)2 | K2CO3 | PPh3 | DMF | 58 |
| 5 | None | K2CO3 | PPh3 | DMF | 0 |
| 6 | Pd(OAc)2 | K2CO3 | PPh3 | MeCN | 28 |
| 7 | Pd(OAc)2 | K2CO3 | PPh3 | DMSO | 57 |
| 8 | Pd(OAc)2 | Cs2CO3 | PPh3 | DMF | 68 |
| 9 | Pd(OAc)2 | Ag2CO3 | PPh3 | DMF | 52 |
| 10 | Pd(OAc)2 | K2CO3 | None | DMF | 15 |
| 11 | Pd(OAc)2 | K2CO3 | P(o-tol)3 | DMF | 67 |
| 12 | Pd(OAc)2 | K2CO3 | dppf | DMF | 54 |
| 13 | Pd(OAc)2 | K2CO3 | Xantphos | DMF | 79 |
| 14 | Pd(OAc)2 | K2CO3 | 1,10-Phen | DMF | 44 |
| 15c | Pd(OAc)2 | K2CO3 | Xantphos | DMF | 63 |
| 16d | Pd(OAc)2 | K2CO3 | Xantphos | DMF | 77 |
Gratifyingly, xantphos (4,5-bis(diphenylphosphino)-9,9-dimethyl xanthene) was found to be the most effective ligand to afford 4a in 79% yield (entry 13). Further exploration of bidentate ligand, such as 1,10-phen provided poor yield of 4a (entry 14). Next, the effect of catalyst loading was investigated and we found that reduction of the catalyst to 2.5 mol% led to decreased product yield (entry 15). The use of higher catalyst loading could not further increase the yield of 4a (entry 16, Table 1). Our attempt to perform the optimized reaction of purified imine derivative 3a in presence of one equivalent of water afforded the pyrazolo[3,4-b]pyridine 5a.
Using the optimized reaction condition (entry 13, Table 1), we evaluated the scope of the reaction by using different acyclic imines 3b–e (Table 2). As shown in Table 2, various acyclic β-bromovinyl imines that have β-substituents such as 4-methylphenyl, 4-chlorophenyl and 3-nitrophenyl groups underwent the above coupling reaction smoothly to give 1,3,4-trisubstituted pyrazolo[3,4-b]pyridines 4b–e in 65–83% yields. Notably, chloro and nitro groups were compatible with the reaction conditions, though they provided slightly less yields. In addition, the coupling reaction of β-bromovinyl imines having β-heterocycles proceeded efficiently to afford 71–74% yields of 4-heterocycle substituted pyrazolo[3,4-b]pyridines 4f–g. Similar intramolecular coupling reactions of cyclic β-bromovinyl imines that have N-alkyl/aryl substituents, under the optimized condition, afforded tetrahydronaphthalene ring fused pyrazolo[3,4-b]pyridine derivatives 4h–i in 75–78% yields. Finally, we explored the scope of this Heck reaction by using different aromatic imines 3j–p under the optimized conditions. The aromatic imines (3j–p) with functional groups such as methoxy, 1,3-dioxole and fluoro groups on the aromatic ring were compatible with the reaction condition to afford 3H-pyrazolo[3,4-c]isoquinolines (4l–p) in 61–75% yields. The formation of compound 4 was finally proved by comparison of spectral and physical data with those reported in the literature for compound 4l.3a Moreover, the Heck reaction of pyridyl imine derivatives 3q–r proceeded smoothly under the optimized reaction conditions to give the corresponding 3H-pyrazolo[4,3-f][1,7]naphthyridines in 68–69% yields. It was worth noting that this intramolecular coupling reaction is highly regioselctive. Although there was a possibility for the formation of nitrogen containing eight membered heterocycle 6a (Scheme 2), it failed to afford it probably, due to the inherent strain of the eight membered ring 6a.
| a Reaction conditions: imine 1 (1.0 mmol), Pd(OAc)2 (5 mol%), PPh3 (10 mol%), K2CO3 (3.0 mmol) in DMF (3.0 mL) was heated at 120 °C for 12 h; isolated yields. |
|---|
 |
On the basis of the above observations and literature reports,10 a plausible reaction mechanism for the Pd-catalyzed formation of compound 4 is shown in Scheme 3. First, oxidative addition of the Pd(0) species to compound 3 forms Pd(II) intermediate A. Then, intramolecular coordination of the olefin of the pyrazole part to the palladium center, followed by migratory insertion of the alkene into the carbon–palladium bond provides intermediate B. The subsequent β-hydride elimination provides compound 4 and a palladium(II)-hydrido complex HPdL2Br, which is reduced by base to afford PdL2 to complete the catalytic cycle.11
Conclusions
In conclusion, we have demonstrated an efficient new approach for the synthesis of biologically important 1,3,4-trisubstituted pyrazolo[3,4-b]pyridines, tetrahydronaphthalene ring fused pyrazolo[3,4-b]pyridines, 3H-pyrazolo[3,4-c]isoquinolines and 3H-pyrazolo[4,3-f][1,7]naphthyridines via an intramolecular Heck reaction. A wide variety of imine derivatives of β-bromovinyl/aryl aldehydes and 5-aminopyrazoles undergo this regioselective reaction to afford good yields of these heterocycles.Acknowledgements
We are grateful to the Director, CSIR-NEIST for his keen interests. This work was financially supported by CSIR-OSDD (HCP-0001), CSIR-ACT (CSC0301) and CSIR-ORIGIN (CSC0108) projects.References
- (a) G. L. Beutner, J. T. Kuethe, M. M. Kim and N. Yasuda, J. Org. Chem., 2009, 74, 789–794 CrossRef CAS PubMed; (b) M. Tandon, M. S. Ali, M. Ashwell, J. Wang, N. Namdev, J. Hill, N. Westlund, A. Dalton, C. Brassard, A. Filikov, R. Palma and D. Vensel, PCT Int. Appl., O2010078427, Chem. Abstr. 2010, 153, 174966.
- (a) J. P. Stasch, E. M. Becker, C. Alonso-Alija, H. Apeler, K. Dembowsky, A. Feurer, R. Gerzer, T. Minuth, E. Perzborn, U. Pleiss, H. Schroder, W. Schroeder, E. Stahl, W. Steinke, A. Straub and M. Schramm, Nature, 2001, 410, 212–215 CrossRef CAS PubMed; (b) B. A. Meiners and A. I. Salama, Eur. J. Pharmacol., 1982, 78, 315–322 CrossRef CAS; (c) L. Revesz, E. Blum, F. E. Di Padova, T. Buhl, R. Feifel, H. Gram, P. Hiestand, U. Manning, U. Neumann and G. Rucklin, Bioorg. Med. Chem. Lett., 2006, 16, 262–266 CrossRef CAS PubMed; (d) F. Manetti, S. Schenone, F. Bondavalli, C. Brullo, O. Bruno, A. Ranise, L. Mosti, G. Menozzi, P. Fossa, M. L. Trincavelli, C. Martini, A. Martinelli, C. Tintori and M. Botta, J. Med. Chem., 2005, 48, 7172–7185 CrossRef CAS PubMed; (e) J. Witherington, V. Bordas, A. Gaiba, N. S. Garton, A. Naylor, A. D. Rawlings, B. P. Slingsby, D. G. Smith, A. K. Takle and R. W. Ward, Bioorg. Med. Chem. Lett., 2003, 13, 3055–3057 CrossRef CAS; (f) S. Wenglowsky, L. Ren, K. A. Ahrendt, E. R. Laird, I. Aliagas and B. Alicke, et al., ACS Med. Chem. Lett., 2011, 2, 342–347 CrossRef CAS PubMed.
- (a) S. L. Bogza, K. I. Kobrakov, A. A. Malienko, I. F. Perepichka, S. Yu. Sujkov, M. R. Bryce, S. B. Lyubchik, A. S. Batsanov and N. M. Bogdan, Org. Biomol. Chem., 2005, 3, 932–940 RSC; (b) B. Jiang, W. Fan, M.-Y. Sun, Q. Ye, S.-L. Wang, S.-J. Tu and G. Li, J. Org. Chem., 2014, 79, 5258–5268 CrossRef CAS PubMed; (c) N. K. Anand, C. M. Blazey, O. J. Bowles, J. Bussenius, S. Costanzo, J. K. Curtis, L. Dubenko, A. R. Kennedy, R. G. Khoury and A. I. Kim, et al.PCT Int. Appl., WO 2005009389 A2, Feb 03, 2005; (d) S. L. Bogza, A. A. Malienko, S. Y. U. Sujkov, I. F. Perepichka, N. M. Bogdan, V. I. Dulenko, K. I. Kobrakov and M. R. Bryce, J. Heterocycl. Chem., 2001, 38, 523–525 CrossRef CAS.
- For selected examples of substituted pyrazolo[3,4-b]pyridines syntheses see: (a) V. A. Chebanov, V. E. Saraev, S. M. Desenko, V. N. Chernenko, I. V. Knyazeva, U. Groth, T. N. Glasnov and C. O. Kappe, J. Org. Chem., 2008, 73, 5110–5118 CrossRef CAS PubMed; (b) V. A. Chebanov, V. E. Saraev, S. M. Desenko, V. N. Chernenko, S. V. Shishkina, O. V. Shishkin, K. M. Kobzar and C. O. Kappe, Org. Lett., 2007, 9, 1691–1694 CrossRef CAS PubMed; (c) S.-L. Wang, Y.-P. Liu, B.-H. Xu, X.-H. Wang, B. Jiang and S.-J. Tu, Tetrahedron, 2011, 67, 9417–9425 CrossRef CAS PubMed; (d) W.-J. Hao, X.-P. Xu, H.-W. Bai, S.-Y. Wang and S.-J. Ji, Org. Lett., 2012, 14, 4894–4897 CrossRef CAS PubMed; (e) Y.-L. Zhong, M. G. Lindale and N. Yasuda, Tetrahedron Lett., 2009, 50, 2293–2297 CrossRef CAS PubMed; (f) S. Lee and S. B. Park, Org. Lett., 2009, 11, 5214–5217 CrossRef CAS PubMed; (g) J. Quiroga, J. Trilleras, B. Insuasty, R. Abonia, M. Nogueras and J. Cobo, Tetrahedron Lett., 2008, 49, 2689–2691 CrossRef CAS PubMed.
- K. Shekarrao, P. P. Kaishap, V. Saddanapu, A. Addlagatta, S. Gogoi and R. C. Boruah, RSC Adv., 2014, 4, 24001–24006 RSC.
- G. Pandey, S. Bhowmik and S. Batra, Org. Lett., 2013, 15, 5044–5047 CrossRef CAS PubMed.
- For selected examples of Pd-catalyzed intramolecular Heck reaction: (a) D. Alberico, N. E. Scott and M. Lauten, Chem. Rev., 2007, 107, 174–238 CrossRef CAS PubMed; (b) J. Pereira, M. Barlier and C. Guillou, Org. Lett., 2007, 9, 3101–3103 CrossRef CAS PubMed; (c) D. Sarlah, P. G. Bulger and K. C. N. Nicolaou, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef PubMed; (d) A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945–2964 CrossRef CAS PubMed; (e) L.-C. Campeau, M. Parisien, A. Jean and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 581–590 CrossRef CAS PubMed; (f) S. Murru, B. McGough and R. S. Srivastava, Org. Biomol. Chem., 2014, 12, 9133–9138 RSC.
- (a) Z. Xiang, T. Luo, K. Lu, J. Cui, X. Shi, R. Fathi, J. Chen and Z. Yang, Org. Lett., 2004, 6, 3155–3158 CrossRef CAS PubMed; (b) L. Zhao, Z. Li, L. Chang, J. Xu, H. Yao and X. Wu, Org. Lett., 2012, 14, 2066–2069 CrossRef CAS PubMed; (c) H. Yuan, K.-J. Bi, B. Li, R.-C. Yue, J. Ye, Y.-H. Shen, L. Shan, H.-Z. Jin, Q.-Y. Sun and W.-D. Zhang, Org. Lett., 2013, 15, 4742–4745 CrossRef CAS PubMed.
- (a) S. Hernández, R. SanMartin, I. Tellitu and E. Domínguez, Org. Lett., 2003, 5, 1095–1098 CrossRef PubMed; (b) J. J. Li, J. Org. Chem., 1999, 64, 8425–8427 CrossRef CAS; (c) P. Goswami, A. J. Borah and P. Phukan, J. Org. Chem., 2015, 80, 438–446 CrossRef CAS PubMed; (d) S. J. Danishefsky, J. J. Masters, W. B. Young, J. T. Link, L. B. Snyder, T. V. Magee, D. K. Jung, R. C. A. Isaacs, W. G. Bornmann, C. A. Alaimo, C. A. Coburn and M. J. Di Grandi, J. Am. Chem. Soc., 1996, 118, 2843–2859 CrossRef CAS; (e) S. Ma and B. Ni, J. Org. Chem., 2002, 67, 8280–8283 CrossRef CAS PubMed; (f) A. Martins, U. Marquardt, N. Kasravi, D. Alberico and M. Lautens, J. Org. Chem., 2006, 71, 4937–4942 CrossRef CAS PubMed; (g) N. D. Buezo, O. G. Mancheño and J. C. Carretero, Org. Lett., 2000, 2, 1451–1454 CrossRef CAS PubMed.
- (a) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS PubMed; (b) D. Xu, C. Lu and W. Chen, Tetrahedron, 2012, 68, 1466–1474 CrossRef CAS PubMed; (c) L. Zhao, Z. Li, L. Chang, J. Xu, H. Yao and X. Wu, Org. Lett., 2012, 14, 2066–2069 CrossRef CAS PubMed.
- (a) G. Satyanarayana and M. E. Maier, J. Org. Chem., 2008, 73, 5410–5415 CrossRef CAS PubMed; (b) D. Bankston, F. Fang, E. Huie and S. Xie, J. Org. Chem., 1999, 64, 3461–3466 CrossRef CAS PubMed; (c) E. L. Cropper, A. J. P. White, A. Ford and K. K. Hii, J. Org. Chem., 2006, 71, 1732–1735 CrossRef CAS PubMed; (d) M.-P. Denieul, B. Laursen, R. Hazell and T. Skrydstrup, J. Org. Chem., 2000, 65, 6052–6060 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedure, characterization data, copies of 1H and 13C NMR spectra for compounds 4a–r, 3a, 3d, 3g, 3i, 3k, 3m, 3o and 3r. See DOI: 10.1039/c4ra17190j |
| This journal is © The Royal Society of Chemistry 2015 |