High temperature stationary phase of polysiloxane containing N,N′-bis(diphenylsilyl) tetramethylcyclodisilazane for gas chromatography
Abstract
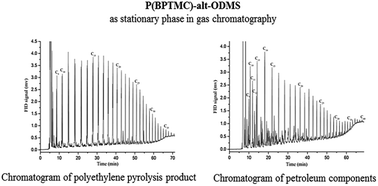
An alternate copolymer (P(BPTMC)-alt-ODMS) constructed from N,N′-bis(diphenyl-silyl) tetramethylcyclodisilazane (BPTMC) and oligo-dimethylsiloxane (ODMS) segments was synthesized and coated on the inside of fused-silica capillary columns to evaluate their properties as stationary phase in gas chromatography. The thermal stability, selectivity, column efficiency, and working range of these capillary columns were characterized. The obtained results indicated that such produced columns exhibited better separation efficiency than commercial ones with high thermal stability and low column bleeding. The upper working temperature can reach up to 400 °C, which resulted in high-quality GC analysis of polyethylene pyrolysis products, petroleum, and aromatic hydrocarbon mixtures. The influence of BPTMC content on the thermal stability and polarity of the stationary phase was also investigated.

 Please wait while we load your content...
Please wait while we load your content...