Solid lipid nanoparticles (SLNs): delivery vehicles for food bioactives
N. P. Aditya and
Sanghoon Ko*
Department of Food Science and Technology, Sejong University, 98 Gunja-dong, Gwangjin-gu, Seoul 143-747, Korea. E-mail: sanghoonko@sejong.ac.kr; Fax: +82 2 3408 4319; Tel: +82 2 3408 3260
First published on 25th March 2015
Abstract
Bioactives which are isolated from different sources like plants, animals, etc. are known to be ideal candidates to treat and prevent chronic health problems such as obesity, hypertension, cardiovascular diseases, cancer, etc. Unfortunately, due to changes in life style and food habits, humans are consuming fewer healthy bioactives than recommended. Hence there is an increasing demand from consumers for food products which are fortified with these bioactives. However, addition of these healthy bioactives to food products for fortification is a challenging job. The main cause is their susceptibility to a complex matrix present in the food product and gastro intestinal tract (GIT) such as pH, temperature, enzymes, etc. Though colossal effort has been made by researchers to successfully translate drug delivery technology for bioactives delivery to protect these susceptible bioactives during production, storage and consumption, success is limited. Due to their near impeccable suitability for the delivery of bioactives in terms of toxicity, scalability, acceptability, solid lipid nanoparticles (SLNs) are drawing attention from food technologists. In this review, effort has been made to present an overview about expectations and suitability of SLNs for delivery of bioactives, selection of the ingredients and their importance in achieving those expectations and an industrially feasible methodology for production of SLNs.
1. Introduction
As quoted by Hippocrates, the father of modern medicine “Let food be thy medicine and medicine be thy food”, in the last few years there has been a growing realization of the pivotal link between diet, health and well-being.1 Thus consumer prefers food products which are tasty and healthy. Further, consumers expect food to provide some additional physiological functions for promoting general body development and disease prevention. These attributes required from the food are provided by bioactive molecules which possess biological activity in addition to their nutritional value.2Bioactive molecules occur naturally in plant and animal products, normally at very low concentrations. Hence fortification is highly desired to increase the concentration of specific and desired bioactives in food products.3 Unfortunately, direct addition of these healthy bioactives to food products results in the unwanted change in the organoleptic properties of food products (e.g. bad odor and taste, undesired mouthfeel) and also destabilizes the product by forming aggregates and sediments. Even then, due to their pharmacokinetic mismatch (lessened stability in gastro intestinal tract (GIT) and absorption variability) a consumer doesn't get full benefits after consumption of food products without their desired organoleptic attributes.1,4
The use of encapsulation technology which is originally used in pharmaceutical industries to increase the stability and bioavailability of drug molecules is also has been in use in food sector for several years for similar purposes with required modification.1,5,6 Encapsulation can be defined as “Processes of entrapping sensitive materials into the matrix of the carrier material which forms the protecting wall and are generally resistant to the sensitive environment for which entrapped materials are not”.7,8 Encapsulation processes may result in the formation of carriers from micro- to nanometer size depending on various factors which are used for their fabrication (e.g. energy input, presence of surface acting materials, core and wall material physicochemical properties etc.).9 In this regard, since last two decades, food scientists and industries are thriving hard to develop novel delivery systems to carry food bioactives which can be used to fortify food products with desired bioactives or combination of bioactive molecules. Problems which are associated with food bioactives are shown in Fig. 1.
 | ||
| Fig. 1 Problems associated with food bioactives for attaining optimal product stability and in body performance. | ||
The main objectives of this review is to provide insight into the expectations from bioactive delivery systems and suitability of solid lipid nanoparticles (SLNs) to fulfill those expectations and to describe how lipid nanostructures can be engineered to meet those expectations.
2. Expectations from bioactives encapsulation technology
Ideally the developed encapsulation system to deliver bioactives is expected to possess the following properties:(A) It is suitable to entrap bioactives with different physicochemical properties (e.g. melting point, solubility, stability, origin etc.) in maximum quantity.
(B) It has capacity to deliver the entrapped cargos to the right place in right time and right concentration.
(C) It can protect the entrapped bioactives both from adverse environment (temperature, oxygen, light etc.) in the complex matrix of the food products and during consumption until entrapped bioactives reaches the desired site of action (pH, ionic strength, enzymes and microbes etc.).
(D) It should prove to be affordable when cost to benefit ratio is considered.
(E) It should be versatile enough to allow its use in different types of food products (liquids, powders, gels etc.).
(F) It should not interfere with desired qualities of the final food products (taste, odor, appearance, viscosity etc.).
(G) It should be easy to prepare but stable in complex processing and storage conditions to use in processed food products.
(H) It should be easily scalable.
(I) It should be stable upon sterilization using methods which are generally used to sterilize food products (thermal treatments, UV sterilization etc.).
(J) Raw materials for fabrication should be easily available in abundance.
(K) It should be stable in various food formats (e.g. liquid, solid, gel etc.). Thus it needs to be stable in aqueous dispersions and also upon lyophilization and spray drying.
In order to obtain above mentioned characteristics from the designed delivery systems, the major determining factors are
(A) Selection of carrier materials and
(B) Production method.
In this review, each of these aspects will be discussed in detail.
3. Selection of carrier materials
Variety of materials is available to use as carrier materials to obtain the desired characteristics as mentioned above. However limitations among them are certified as “generally recognized as safe” (GRAS) which can be added to food products to obtain the desired functional attributes like controlled and targeted delivery, taste and odor masking and to protect from degradation etc.1,10 Further it is noteworthy that due to preference for fully natural materials as carrier materials among the consumers and producers, several GRAS approved materials which are used in drug delivery are not preferred for use in food sector to deliver bioactives. In addition, presence of non food particles in food products in large quantity also compromises nutritional value of products.11 Hence, in food sector, delivery systems which are comprised of natural food additives are preferred.Important factors which need to be considered before selecting the carrier material are,
(A) Interaction between functional ingredient and carrier material (carrier and bioactive interaction),
(B) Intended use (products in which it needs to be added and processing and storage condition of that particular products etc.),
(C) Effect of the carrier material on the consistency and the structure of the product,
(D) Presence of other co-excipients presence in the system and their interaction behavior with the carrier materials,
(E) Biological barriers which should be overcome in order to obtain desired biological activity,
(F) Toxicity upon chronic ingestion.
3.1. Lipids and lipid based delivery systems
Lipids are the group of natural molecules which include mono-, di- and triglycerides, fats, waxes, sterols, phospholipids etc.12 These lipids are important constituents for existence of life. Starting from the fetal growth they are required in all stages of life to perform specific activity or to maintain general health of the organism. In living organism they provide energy, act as reserve source of energy and aid the absorption of fat soluble vitamins, nutraceuticals and drug molecules.13 Lipids play important role as the components which give desired qualities like texture, aroma, color, flavor, satiety, mouthfeel and rheological properties to food products.14Thus due to their high acceptability, non toxicity and suitability to use in food products, lipid based delivery systems are rapidly gaining interest among the food scientists for application in food systems to deliver bioactives.9,10 When referring to lipid based delivery systems, it includes liposomes, solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), emulsions, micelles etc.15 In this article, we will provide an overview about SLN which is the most rapidly developing carrier systems among lipid based delivery systems for food application due to their inherent characteristic i.e. stability in complex systems and ability to withhold the bioactives within the core of the nanoparticles which suit food application. These characteristics are less seen in other lipid based delivery systems like liposomes and emulsions (Fig. 2). Due to high cost and reports of toxicity, synthetic polymeric nanoparticles are less preferred in food delivery compared to drug delivery systems.16
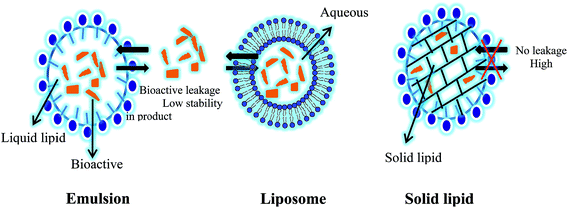 | ||
| Fig. 2 Comparison between lipid based delivery systems with regarding to their stability in complex food matrix and gastro intestinal track (GIT). | ||
3.1.2.1. Production. (A) SLNs can be fabricated using methods like high pressure homogenization (HPH) and microfluidization which can be easily scalable for industrial production. Several lipid based nanoparticle formulations are in the market for drug and cosmeceutical delivery.
(B) SLNs can be fabricated without using organic solvents which otherwise are not acceptable to use in food products.
(C) SLNs can be sterilized using techniques which are used to sterilize food products (heat sterilization, UV sterilization etc.).
(D) SLNs can be obtained both in the liquid and solid forms. Hence they are easy to use both in solid and liquid food products.
3.1.2.2. Acceptability. (1) Lipids, which are used to fabricate SLNs are non toxic and biocompatible. Several food grade materials are available (e.g. tristearin is FDA approved food additive [21CFR172.811]).
(2) Raw materials used in the production of SLNs such as lipids, surfactants and co-surfactants are easily available in large scale with preferred quality.
(3) Food grade surfactants like polyoxyethylene sorbitan monooleate (e.g. Tween 80), proteins etc., can be used to fabricate SLNs.
(4) In order to be best suited for oral delivery, this is the preferred route of administration for nutraceuticals.
3.1.2.3. Biological activity. (1) In order to be best suited for oral delivery, this is the preferred route of administration for nutraceuticals.
(2) SLN provide chemical protection to the encapsulated nutraceuticals against chemical degradation in the product (pH, salt, temperature etc.) during storage.
(3) SLN protects susceptible nutraceuticals against degradation in GIT.
(4) SLN provide chemical protection to the encapsulated nutraceuticals against chemical degradation in the product (pH, salt, temperature etc.) during storage.
(5) SLN enhances bioaccessibility of nutraceuticals in the intestine by forming micelles.
(6) Rate and site of nutraceutical release can be controlled by varying SLN size and composition (surfactant, co-surfactant etc.).
In the next part of this article, an important aspect which needs to be considered before fabricating SLNs to obtain optimal benefit such as various industrially feasible methods for the production, advantages and disadvantages with regarding their application in food products are discussed in detail.
3.2. Thing to be considered in the formulation of SLNs for bioactives delivery
Excipients such as lipids, surfactants, co-surfactants, and cryoprotectants etc. which are used to fabricate SLNs determine their fate with regarding to their ability for encapsulation and loading efficiency, targetability, stability etc. Hence selections of proper and compatible excipients are highly desired. The common excipients which are generally used to fabricate SLNs are listed in Table 1. Important factors which need to be reviewed carefully before designing delivery system are as follows.| Items | Food-grade materials | References |
|---|---|---|
| Excipients | Triacylglycerols | |
| Trimyristin (Dynasan 114) | 38 and 54 | |
| Tripalmitin (Dynasan 116) | 17 and 38 | |
| Tristearin (Dynasan 118) | 55 and 56 | |
| Mono-, di- and triglycerides mixtures | ||
| Glyceryl monostearate (Imwitor 900) | 29, 49 and 57 | |
| Glyceryl behenate (Compritol 888 ATO) | 35, 58 and 59 | |
| Waxes | ||
| Bee wax | 60 and 61 | |
| Cetyl palmitate | 62 and 63 | |
| Hard fats | ||
| Stearic acid | 45, 64 and 65 | |
| Palmitic acid | 66 and 67 | |
| Behenic acid | 68 and 69 | |
| Other lipid | ||
| para-Acyl-calix(4)arenes | 70 and 71 | |
| Surfactants (HLB) | Polyoxyethylene sorbitan monooleate | |
| Polysorbate 20 (16.7) | 72 | |
| Polysorbate 60 (14.9) | 73 | |
| Polysorbate 80 (15) | 73 | |
| Lecithin | ||
| Soy and egg lecithin (4.0) | 57, 73 and 74 | |
| Protein | ||
| Whey protein | 75 | |
| Others | ||
| Alkyl polyglucosides | 76 | |
| Amino acids | 27 | |
Important factors needs to be considered before selection of lipids are as follows.
3.2.1.1. Loading and entrapment efficiency. To reduce the concentration of carrier material required to fortify the product with required amount of bioactives which otherwise may interfere with original product quality, it is highly desired to select the carrier material with highest loading efficiency for the desired bioactives. In this regard, it is highly warranted to study the solubility of chosen bioactives in the various lipids with different physicochemical characteristics.22 Although this methodology is quite often used before designing drug delivery systems, mention of this kind of study in food bioactives delivery is rare.23
In general lipophilic nature of the glycerides (e.g. trimyristin, C = 14; tripalmitin, C = 16; tristearin, C = 18) increases with the increase in the hydrocarbon chain length. This increases solubility and loading efficiency of hydrophobic bioactives within the SLNs. Further, lipids with different hydrocarbon chain lengths which are mixtures of mono-, di-, and triglycerides (e.g. glycerylbehenate and glycerylmonostearate, GMS) form nanoparticles with many lattice defects. These defects provide the accommodation for the guest molecules (bioactives) to stay inside the nanoparticle matrix. In one of our studies, maximum curcumin loading and entrapment efficiency was observed in GMS which is composed of mono-, di-, and triglycerides compared to trimyristin (TM) and tristearin (TS) which are composed of >95% triglycerides.24 Recently, we have shown that, in addition to hydrophobicity of lipid and bioactive molecules, molecular weight of the bioactives also plays important role in determining the loading efficiency. Loading efficiency decreases with increase in molecular weight of the bioactives. In case of curcumin and genistein co-loading, though the curcumin (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 3.1) is more hydrophobic than genistein (log
P = 3.1) is more hydrophobic than genistein (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 2.9), genistein loading efficiency was significantly more than curcumin.13 Another important factor which determines the loading efficiency of lipid nanoparticles is recrystallization. If complete recrystallization is not imposed by cooling below lipid critical recrystallization temperature, soon after the formation of SLN during fabrication, instead of solid matrix liquid droplet resembling the emulsion is formed. In this metastable super cooled condition, these nanoparticles loose the advantage of having solid matrix and decreases their loading capacity and capacity to retain the bioactives within the lipid matrix for longer period (Fig. 2).5,24,25
P = 2.9), genistein loading efficiency was significantly more than curcumin.13 Another important factor which determines the loading efficiency of lipid nanoparticles is recrystallization. If complete recrystallization is not imposed by cooling below lipid critical recrystallization temperature, soon after the formation of SLN during fabrication, instead of solid matrix liquid droplet resembling the emulsion is formed. In this metastable super cooled condition, these nanoparticles loose the advantage of having solid matrix and decreases their loading capacity and capacity to retain the bioactives within the lipid matrix for longer period (Fig. 2).5,24,25
Further, for delivery of hydrophilic molecules, instead of SLNs, lipid drug conjugates (complexation of bioactives with lipid molecules) are fabricated first and later they were converted into nanoparticles. This method decreases the requirement of carrier materials.26 Though this technology is used in pharmaceutical industry to deliver various hydrophilic drug molecules, its application in food industry is still not explored. In future it will be worthy to study the lipid bioactive conjugate using bioactives like catechin and rutin which are less soluble in lipid.
3.2.1.2. Stability. Stability is important for successful incorporation of fabricated SLNs into food products. Due to complex nature of food matrix (temperature, pH, ionic strength etc.) and also sterilization methods used to store foods make these nanoparticles vulnerable for degradation by aggregation, coalescence, creaming, and sedimentation. Hence extra care needs to be taken to fabricate SLNs for food application in comparison to pharmaceutical application. On the other hand, shelf life of food products is shorter compared to pharmaceutical products. Hence stability of the SLNs for a few months is sufficient in most of the cases whereas it is expected to be at least two years in SLNs fabricated for drug delivery.
Crystallinity of the lipid plays an important role in determining the stability of the nanoparticles. Fabrication of SLNs using lipids without lattice defects (e.g. monoacid triglycerides like tripalmitate, bee wax, cetylpalmitate) results in the bioactive expulsion from the nanoparticle matrix due to polymorphic transition of unstable α subcells into stable β subcells.27,28 Conversion of α subcell into β subcells abrogates the space located in the matrix of the SLNs which provide accommodation for bioactives. Said so, it is very important to balance the composition of mono-, di-, and triglycerides. Earlier studies have shown that, among the glycerides, tribehenin which composes 15% of monoglycerides shows better stability compared to glycerylmonostearate (∼50% monoglycerides) and tripalmitate (<5% monoglycerides). Monoglycerides possess the surfactant properties and hence stabilizes the SLNs up to certain extent. However above certain concentration, they destabilize the nanostructures.28 Even though lattice defects could be slightly increased by mixing two lipids, trivial increase in loading efficiency and stability was observed.29
Another important factor which determines the stability of SLN is their crystallization rate and temperature. If proper care is not taken during fabrication to induce controlled crystallization in time, then formed nanoparticles become vulnerable for degradation. The uncontrolled crystallization leads to conversion of spherical structures into platelet like structures which lacks the controlled release property and stability.16 Jenning and co-authors reported the variation in the formation of α and β crystals upon changing the cooling temperature. Fast cooling (10 °C min−1) resulted in the formation of predominant α crystals whereas decreasing the cooling rate to 2 °C min−1 resulted in the formation of β crystals.30 Similar results were also published by other research groups.31
In this direction, recently we fabricated SLN stabilized with non ionic surfactant Tween 80 and stability of these SLNs (aqueous stability, dispersibility, flow behavior, size etc.) was studied under various environmental conditions which may exist in food products. Presence of electrolytes (NaCl) resulted in increased viscosity of the SLN suspension. Further increasing NaCl concentration resulted in conversion of Newtonian flow behavior to non-Newtonian flow behavior. Since most of the food products like beverages contain electrolytes in the range of 20 mM, proper selection of surfactants are warranted to avoid the aggregation and gelation of SLNs in such conditions.32 Several other studies also reported similar effect of ionic strength and pH on stability of SLNs.33
With regarding to chemical stability of lipid, lipids with longer hydrocarbon chain length (tristearin) are known to have highest stability (∼4% degradation upon storage). Further, in most cases degradation of lipid was within ∼10%.34 Though, in food sector stability up to 2 years is not expected, these lipid degradation studies were conducted only with regarding to their stability in pharmaceutical formulations. Presence of complex food matrix may alter the stability profile of lipids. Unfortunately, as per our knowledge, no study has been conducted regarding stability of various solid lipids in the complex food structures (various temperature, pH, ionic strength etc.). Hence, there is a need to conduct these studies in the near future to successfully incorporate these SLNs into industrially processed foods.
3.2.1.3. Particle size. Particle size of SLN is an important criterion in designing delivery systems for bioactives for the reason that it affects organoleptic properties like appearance, stability, texture, and mouthfeel etc. and biological performance (dissolution, absorption etc.) of food products.17,35 In this regard, chemical nature (type of fatty acid chain, degree of unsaturation, melting point, shape and surface area of lipid crystals, hydrophilicity etc.) plays an important role.36 From earlier studies it is evident that, lipid with higher melting point forms larger nanoparticles due to increased viscosity.37 Attesting these observations, in one of our studies, curcumin loaded SLNs fabricated using higher melting point lipid resulted in the formation of bigger SLNs compared to SLNs fabricated using the lipids with lower melting point. SLN fabricated using tristearin (MP: 70 °C) had the size of 314 ± 23 nm compared to SLNs fabricated using glycerylmonostearate (MP: 56 °C) which had the size of 111 ± 3 nm.38 Further, with regarding to its application in food products, recent studies from our laboratory and others have shown that gradual increase in electrolyte concentration resulted in particle growth in Tween 80 stabilized SLN. This is evident by size increase of the SLNs. However pH of the solution has little effect on the stability of these SLNs.32
3.2.1.4. Bioavailability. In general, bioavailability refers to the amount of bioactives available to act at the site of physiological activity in the living system. Lipid nanoparticles are used to increase oral bioavailability of poorly water soluble bioactives such as curcumin, quercetin etc. and bioactives which has low cell permeability like catechin.39
Though, SLN digestion is a complex process, it can be categorized into three main phases, (1) digestive phase; (2) absorption phase and (3) entering the blood circulation.40 In the beginning digestion is initiated by the hydrolysis of triglycerides (TGs) in to diglyceride (DGs) and fatty acids (FFAs) by gastric and lingual lipases in the stomach (Fig. 3). Further, shear force generated during antral contraction, retropulsion and gastric emptying results in the formation of emulsions from the hydrolyzed lipids. In the duodenum, presence of lipids stimulates the release of bile salts, pancreatic fluids and binary lipids from gall bladder which further assists in the formation emulsions. In addition, bile salts which are amphiphilic in nature (one side is hydrophilic and another side of the same molecule is hydrophobic) acts on this lipids and align themselves to face their hydrophobic part towards the lipid droplets and forms the layer around droplets. This increases lipid droplets surface area and hydrophilicity and also avoids their re-aggregation. Here the lipase which is a hydrophilic enzyme acts on the remaining triglycerides and diglycerides to break apart glycerol back bone and fatty acid chains by breaking the bond which link them. As a result from each triglyceride 2 fatty acids and one monoglycerides (MGs) are released.41 This released FFAs and MGs will form different structures like micelles, mixed micelles, vesicles etc. Here the bioactive compounds will be trapped inside these structures. The presence of bile salts increase their solubility in lumen of the gut (>1000 fold) due to which required concentration gradient for the diffusion of these structures into enterocytes is generated.42 While taking up these structures, bioactives which were trapped inside these structures will also get absorbed. In the cytosol (cell compartment) absorbed bioactive compounds will directly go to portal circulation if they have the sufficient solubility (p < 5). In case of bioactives which are highly hydrophobic (p > 5), add on to the intestinal lipoproteins (chylomicrons) in the enterocytes which were formed by the re-esterification of MGs and FFAs into TGs either by monoacyl glycerol pathway or phosphatidic pathway. Here these chylomicrons fuses with basolateral cell membrane of the enterocytes and transported to the circulation through thoracic lymph duct.
 | ||
| Fig. 3 Schematic representation of digestion and absorption of bioactive compounds during oral intake using solid lipid nanoparticles (SLN). | ||
As in other factors, lipid composition plays an important role in determining the bioavailability pattern of entrapped nutraceutical molecules. In one of the recent studies from our laboratory it was found that lipid nanocarriers like NLCs which are composed of lipids with medium hydrocarbon chain length (C = <12) has increased the bioavailability of hydrophobic bioactives such as quercetin compared to SLNs which are fabricated using long hydrocarbon chain length (C = 21). This could be attributed to the formation of medium chain free fatty acids after digestion which would migrate to the surrounding aqueous phase without interfering in the lipase activity resulting in the increased bioavailability. Whereas in case of long chain triglycerides (C = 21), formation of long chain free fatty acids after digestion accumulates in the oil and water interface resulting in lipase activity inhibition and reduced bioavailability.43
3.2.2.1. Surfactant type. One among the key challenges for the successful stabilization of SLNs in complex food matrix is selection of suitable food grade surfactant. Combinations of ionic and non ionic surfactants are used to obtain matrix stability and suspension stability of SLNs in drug formulations. However for food application, ionic surfactants are not preferred due to toxicity issues.32 Though biopolymers are non toxic their use in food is restricted due to their high emulsifying ability which induces gelling during storage.10 Hence proteins and polyoxyethylene sorbitan monooleate (Tweens) are preferred surfactants.
The selection of surfactant for the particular formulation depends on lipid matrix type and intended use. The surfactant property which determines the ability to act as surface active agents are molecular weight, chemical structure, hydrophile–lipophile balance (HLB), surface charge etc. Surfactants contain both hydrophilic and a lipophilic elements which assist them to align them in W/O, O/W or water-in-oil-in-water (W/O/W) interface. Selections of surfactants are usually done by calculating their HLB value. HLB is given by the balance between the size and strength of the hydrophilic and the lipophilic groups in a surfactant molecule.36 According to Griffin classification, surfactants which has the HLB value <9 are hydrophobic, <11 are hydrophilic and in between them are in between.17 Usually HLBs are preferred by deciding type of interface which needs to be fabricated. In general HLB 3.5–6 supports formation of W/O emulsion and HLB 8–18 results in O/W emulsion. However, different types of lipids require different HLBs in order to ensure formation of stable nanoparticles.44 Severino et al. reported superior stability of SLNs with the combination of polysorbate 80 and sorbitan trioleate due to their structure compatibility.45 Another important aspect about the use of surfactants in SLN fabrication is controlling crystallization processes. An amphiphilic molecule with high hydrophilicity covers the new surfaces which are emerged due to conversion of α crystal to β crystals during storage (polymeric transition). In pharmaceutical formulation, recrystallization is inhibited by using bile salts like sodium glycocholate, sodium taurodeoxycholate etc. However in food products these bile salts are not preferred due to cost constraints and their bitter taste.46,47
In summary, ideal surfactant needs to stabilize the SLN against physical instability as well as to modulate crystallization (to prevent recrystallization). To achieve this in the formulations, combinations of surfactants with synergistic activity are used. Generally non ionic surfactants which provide stabilization against recrystallization are used in combination with ionic surfactants which stabilize SLN by avoiding aggregation and flocculation. Further, combination of surfactants efficiently covers the surface area as well as produces sufficient viscosity to promote the nanoparticle stability.48 Since, ionic surfactants are not preferred in food product; highly biocompatible molecules which can provide both suspension stability and matrix stability are warranted.
Recently, Salminen and co-workers reported significant increase in stability of aromatic amino acids stabilized SLNs with regarding to the retention of α crystals compared to SLN stabilized with other two synthetic surfactants such as Pluronic F68, Tween 60 and 80. The main reason for their superior stability is due to the presence of both hydrophilic and hydrophobic areas with high water solubility. These characteristics allow the surfactant molecules to cover the new area formed during conversion of α crystal to β crystal. Recently in our laboratory we successfully stabilized SLN using β-lactoglobulin; the fabricated SLNs showed particle stabilization and matrix stabilization for more than 30 days in room temperature (22 ± 3 °C). This is due to the presence of essential characteristics in β-lactoglobulin to become an ideal surfactant (amphiphilic with pronounced hydrophobic areas, but highly water soluble). In the near future, stability of these β-lactoglobulin stabilized SLN will be studied in complex food matrix (unpublished data). Recent study from our laboratory revealed that Tweens successfully stabilizes the SLN in various pH conditions. However presence of electrolytes destabilized the SLN stabilized by Tweens.
3.2.2.2. Surfactant concentration. Presence of number of surfactant molecules on the surface area of the nanoparticles also affects the stability of SLNs. Presence of sufficiently high number of surfactants during the formation of new surface area after conversion of bulk lipids in to SLNs ensures stabilization by decreasing surface tension. Further availability of a few excess molecules of surfactant also effectively decreases the conversion of unstable α crystals of lipids into more thermodynamically stable β form during the storage.27,32 Said so, its concentration should be optimum. Excess use of surfactant results in decreased loading efficiency and induces burst release.48
4. Production of SLNs
There are several industrially feasible methods are available to fabricate the SLNs. Generally SLNs are fabricated using either top down or bottom up methods as shown in Fig. 4.Some of the methods which are used in fabricating the bioactives loaded SLNs for food application are listed below.
(A) Top down technology
(a) High pressure homogenization (HPH)49
(b) Microfluidization50
(c) Membrane contactor method51
(B) Bottom up technology
(a) Emulsification–solvent evaporation52
(b) Emulsification–solvent diffusion53
In principle, selection of method depends on the physicochemical characteristics of the excipients and bioactives, quantity of production etc. Among the above mentioned methods, HPH and microfluidization are the most preferred ones due to their ease in scalability and non toxicity. Hence HPH and microfluidization which are industrially most feasible methods are explained below in detail.
4.1. High pressure homogenization (HPH)
Initially this method has been used to fabricate nanoemulsion for parenteral nutrition production.9 In food industry, this technique is quite often used to improve the stability, shelf life, taste and quality of food products. Thus HPH is highly reliable and powerful technique to produce SLNs for food application. In HPH, lipid and bioactive melt is passed through the valve gap where required operating pressure (100–2000 bar) can be created by piston pump. Here it breaks up the oil and water phases and form small W/O or O/W emulsion due to high shear stress and cavitation forces. After repeatedly exposing the oil and water phases to high shear stress and cavitation forces, emulsion with required size can be obtained. The factors which mainly determine the quality of SLN produced by HPH are homogenization pressure, temperature, number of cycles etc.9,16 Homogenization can be done in both hot (melted lipid) and cold (powdered lipid) condition which helps to avoid the degradation of heat sensitive bioactives like lycopene. The disadvantages are its high energy dependence and distribution of bioactives into aqueous phase during homogenization. Fig. 5 illustrates the schematic procedure for fabrication of SLN using HPH. | ||
| Fig. 5 Illustration of solid lipid nanoparticles (SLNs) production by hot and cold homogenization method. | ||
4.2. Microfluidization
Like HPH, microfluidization is a high energy emulsification method used to fabricate both W/O or O/W emulsion. This methodology can be used to fabricate SLNs at both laboratory and industrial scale and it is easily scalable. In food industry, it is used to fabricate emulsions and to homogenize proteins (whey protein, trypsin, milk etc.). In microfluidizer, oil and water phases breakup occurs from high turbulence and shear created by the collision of two impinging jets oriented at 180° to each other. Here also operating pressure up to 150 MPa can be created by using piston pump. Size of the emulsion formed depends on operating pressure and the number of passes. Increasing operating pressure and the number of passes decreases size of the SLNs.The specific advantages of these high energy emulsification methods (HPH and microfluidization) regarding bioactives loaded SLN production are as following
(A) SLN can be produced in both hot and cold conditions which help to avoid the degradation of heat sensitive bioactives like curcumin, quercetin etc., (Fig. 5).
(B) Lipids are food ingredients or additives. These helps to maintain the required organoleptic properties (transparency, mouthfeel) of food products even after the addition of SLNs.
(C) SLNs can be produced without using organic solvents.
(D) No contamination from instruments as in case of high shear homogenization (ultra sonication) occurs.
(E) HPH and microfluidization are easily scalable.
5. Conclusion
Though, SLNs do not fulfill all the criteria to be used as a sole carrier system for bioactives, they have several advantages which include the non toxic composition (physiological compounds), avoidance of organic compounds for production, high entrapment efficiency, well established industrially feasible method for production etc.The main disadvantages are low loading efficiency for hydrophilic bioactives, polymorphic transition during storage and lack of proper evidence for their behavior in complex food matrix etc. However these disadvantages can be successfully avoided by selecting suitable excipients for production, compatible bioactives for incorporation and finally choosing suitable production and storage conditions.
Presence of SLNs in the food products will help to materialize the need of bioactives enriched products which can deliver their health benefits to consumers without losing their nutritional properties as well as increase the profitability of food industries. In the future, bioactives entrapped in SLNs can be expected to take the central stage in the development of fortified food products.
In conclusion, SLN are very multifaceted delivery structure with several advantages and drawbacks to use them as bioactives delivery vehicles. In future, lot of efforts needs to be to put for fabrication of SLN which are particularly tuned for the delivery of bioactives to successfully use them in industrially processed food products.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (no. 2014M3A7B4051125).References
- A. R. Patel and K. P. Velikov, LWT–Food Sci. Technol., 2011, 44, 1958–1964 CrossRef CAS PubMed.
- J. E. Norton, G. A. Wallis, F. Spyropoulos, P. J. Lillford and I. T. Norton, Annu. Rev. Food Sci. Technol., 2014, 5, 177–195 CrossRef CAS PubMed.
- A. Das and C. K. Sen, in Nutraceutical and Functional Food Regulations in the United States and Around the World, ed. D. Bagchi, Academic Press, San Diego, 2nd edn, 2014, pp. 13–39, DOI:10.1016/B978-0-12-405870-5.00002-5.
- M. F. Yao, H. Xiao and D. J. McClements, Annu. Rev. Food Sci. Technol., 2014, 5, 53–81 CrossRef PubMed.
- M. A. Neves, J. Hashemi and C. Prentice, Curr. Opin. Food Sci., 2015, 1, 7–12 CrossRef PubMed.
- E. Acosta, Curr. Opin. Colloid Interface Sci., 2009, 14, 3–15 CrossRef CAS PubMed.
- V. Nedovic, A. Kalusevic, V. Manojlovic, S. Levic and B. Bugarski, Procedia Food Sci., 2011, 1, 1806–1815 CrossRef CAS PubMed.
- F. Tamjidi, M. Shahedi, J. Varshosaz and A. Nasirpour, Innovative Food Sci. Emerging Technol., 2013, 19, 29–43 CrossRef CAS PubMed.
- W. Mehnert and K. Mäder, Adv. Drug Delivery Rev., 2012, 64, 83–101 CrossRef PubMed.
- F. Tamjidi, M. Shahedi, J. Varshosaz and A. Nasirpour, Innovative Food Sci. Emerging Technol., 2013, 19, 29–43 CrossRef CAS PubMed.
- S. Singh, A. K. Dobhal, A. Jain, J. K. Pandit and S. Chakraborty, Chem. Pharm. Bull., 2010, 58, 650–655 CrossRef CAS.
- S. Phan, S. Salentinig, E. Gilbert, T. A. Darwish, A. Hawley, R. Nixon-Luke, G. Bryant and B. J. Boyd, J. Colloid Interface Sci. DOI:10.1016/j.jcis.2014.11.026 , in press..
- N. P. Aditya, M. Shim, I. Lee, Y. Lee, M. H. Im and S. Ko, J. Agric. Food Chem., 2013, 61, 1878–1883 CrossRef CAS PubMed.
- J. B. German, in Impact of Processing on Food Safety, ed. L. Jackson, M. Knize and J. Morgan, Springer US, 1999, vol. 459, ch. 3, pp. 23–50 Search PubMed.
- M. Fathi, M. R. Mozafari and M. Mohebbi, Trends Food Sci. Technol., 2012, 23, 13–27 CrossRef CAS PubMed.
- H. Harde, M. Das and S. Jain, Expert Opin. Drug Delivery, 2011, 8, 1407–1424 CrossRef CAS PubMed.
- P. Severino, T. Andreani, A. S. Macedo, J. F. Fangueiro, M. H. A. Santana, A. M. Silva and E. B. Souto, Drug Delivery, 2012, 2012, 10 Search PubMed.
- M. Trotta, M. E. Carlotti, M. Gallarate, G. P. Zara, E. Muntoni and L. Battaglia, J. Dispersion Sci. Technol., 2011, 32, 1041–1045 CrossRef CAS.
- P. Shahgaldian, M. Cesario, P. Goreloff and A. W. Coleman, Chem. Commun., 2002, 326–327, 10.1039/B111367b.
- J. F. Fangueiro, E. Gonzalez-Mira, P. Martins-Lopes, M. A. Egea, M. L. Garcia, S. B. Souto and E. B. Souto, Pharm. Dev. Technol., 2013, 18, 545–549 CrossRef CAS PubMed.
- K. Ahmed, Y. Li, D. J. McClements and H. Xiao, Food Chem., 2012, 132, 799–807 CrossRef CAS PubMed.
- K. Vivek, H. Reddy and R. S. R. Murthy, AAPS PharmSciTech, 2007, 8, 16–24 CrossRef PubMed.
- N. P. Aditya, S. Aditya, H.-J. Yang, H. W. Kim, S. O. Park, J. Lee and S. Ko, J. Funct. Foods, 2015, 15, 35–43 CrossRef CAS PubMed.
- A. P. Nayak, W. Tiyaboonchai, S. Patankar, B. Madhusudhan and E. B. Souto, Colloids Surf., B, 2010, 81, 263–273 CrossRef CAS PubMed.
- K. Westesen and H. Bunjes, Int. J. Pharm., 1995, 115, 129–131 CrossRef CAS.
- C. Olbrich, A. Gessner, O. Kayser and R. H. Muller, J. Drug Targeting, 2002, 10, 387–396 CrossRef CAS PubMed.
- H. Salminen, T. Helgason, S. Aulbach, B. Kristinsson, K. Kristbergsson and J. Weiss, J. Colloid Interface Sci., 2014, 426, 256–263 CrossRef CAS PubMed.
- V. Jenning and S. H. Gohla, J. Microencapsulation, 2001, 18, 149–158 CrossRef CAS PubMed.
- K. Westesen, B. Siekmann and M. H. J. Koch, Int. J. Pharm., 1993, 93, 189–199 CrossRef CAS.
- V. Jenning, A. F. Thünemann and S. H. Gohla, Int. J. Pharm., 2000, 199, 167–177 CrossRef CAS.
- J. Weiss, E. A. Decker, D. J. McClements, K. Kristbergsson, T. Helgason and T. Awad, Food Biophys., 2008, 3, 146–154 CrossRef.
- K. O. Choi, N. P. Aditya and S. Ko, Food Chem., 2014, 147, 239–244 CrossRef CAS PubMed.
- Z. G. Cui, K. Z. Shi, Y. Z. Cui and B. P. Binks, Colloids Surf., A, 2008, 329, 67–74 CrossRef CAS PubMed.
- A. Radomska-Soukharev, Adv. Drug Delivery Rev., 2007, 59, 411–418 CrossRef CAS PubMed.
- V. Kakkar, S. Singh, D. Singla and I. P. Kaur, Mol. Nutr. Food Res., 2011, 55, 495–503 CAS.
- D. J. Hauss, Adv. Drug Delivery Rev., 2007, 59, 667–676 CrossRef CAS PubMed.
- M. Uner and G. Yener, Int. J. Nanomed., 2007, 2, 289–300 CAS.
- A. P. Nayak, W. Tiyaboonchai, S. Patankar, B. Madhusudhan and E. B. Souto, Colloids Surf., B, 2010, 81, 263–273 CrossRef CAS PubMed.
- N. P. Aditya, S. Aditya, H. Yang, H. W. Kim, S. O. Park and S. Ko, Food Chem., 2015, 173, 7–13 CrossRef CAS PubMed.
- L. H. Reddy and R. S. Murthy, Indian J. Exp. Biol., 2002, 40, 1097–1109 CAS.
- Y. Sun, Z. Xia, J. Zheng, P. Qiu, L. Zhang, D. J. McClements and H. Xiao, J. Funct. Foods, 2015, 13, 61–70 CrossRef CAS PubMed.
- H. Westergaard and J. M. Dietschy, J. Clin. Invest., 1976, 58, 97–108 CrossRef CAS PubMed.
- L. Salvia-Trujillo, C. Qian, O. Martín-Belloso and D. J. McClements, Food Chem., 2013, 139, 878–884 CrossRef CAS PubMed.
- T. Schmidts, D. Dobler, C. Nissing and F. Runkel, J. Colloid Interface Sci., 2009, 338, 184–192 CrossRef CAS PubMed.
- P. Severino, S. C. Pinho, E. B. Souto and M. H. A. Santana, Colloids Surf., B, 2011, 86, 125–130 CrossRef CAS PubMed.
- K. Westesen, H. Bunjes and M. H. J. Koch, J Controlled Release, 1997, 48, 223–236 CrossRef CAS.
- K. Westesen and B. Siekmann, Int. J. Pharm., 1997, 151, 35–45 CrossRef CAS.
- R. Cavalli, O. Caputo, E. Marengo, F. Pattarino and M. R. Gasco, Pharmazie, 1998, 53, 392–396 CAS.
- N. P. Aditya, A. S. Macedo, S. Doktorovova, E. B. Souto, S. Kim, P.-S. Chang and S. Ko, LWT–Food Sci. Technol., 2014, 59, 115–121 CrossRef CAS PubMed.
- L. Lee and I. T. Norton, J. Food Eng., 2013, 114, 158–163 CrossRef CAS PubMed.
- D. M. Lloyd, I. T. Norton and F. Spyropoulos, J. Membr. Sci., 2014, 466, 8–17 CrossRef CAS PubMed.
- M. Fathi, Á. Martín and D. J. McClements, Trends Food Sci. Technol., 2014, 39, 18–39 CrossRef CAS PubMed.
- A. Imbrogno, E. Piacentini, E. Drioli and L. Giorno, J. Membr. Sci., 2014, 467, 262–268 CrossRef CAS PubMed.
- J. Sun, C. Bi, H. M. Chan, S. Sun, Q. Zhang and Y. Zheng, Colloids Surf., B, 2013, 111, 367–375 CrossRef PubMed.
- R. Cortesi, E. Esposito, G. Luca and C. Nastruzzi, Biomaterials, 2002, 23, 2283–2294 CrossRef CAS.
- L. H. Reddy, K. Vivek, N. Bakshi and R. S. R. Murthy, Pharm. Dev. Technol., 2006, 11, 167–177 CrossRef CAS PubMed.
- H. Li, X. Zhao, Y. Ma, G. Zhai, L. Li and H. Lou, J. Controlled Release, 2009, 133, 238–244 CrossRef CAS PubMed.
- K. Teskač and J. Kristl, Int. J. Pharm., 2010, 390, 61–69 CrossRef PubMed.
- S. Jose, S. S. Anju, T. A. Cinu, N. A. Aleykutty, S. Thomas and E. B. Souto, Int. J. Pharm., 2014, 474, 6–13 CrossRef CAS PubMed.
- J. Zhang and E. Smith, J. Pharm. Sci., 2011, 100, 896–903 CrossRef CAS PubMed.
- D. T. Baviskar, A. S. Amritkar, H. S. Chaudhari and D. K. Jain, Pharmazie, 2012, 67, 701–705 CAS.
- S. A. Wissing and R. H. Muller, Pharmazie, 2001, 56, 783–786 CAS.
- L. Montenegro, A. Campisi, M. G. Sarpietro, C. Carbone, R. Acquaviva, G. Raciti and G. Puglisi, Drug Dev. Ind. Pharm., 2011, 37, 737–746 CrossRef CAS PubMed.
- H. Yuan, J. Chen, Y.-Z. Du, F.-Q. Hu, S. Zeng and H.-L. Zhao, Colloids Surf., B, 2007, 58, 157–164 CrossRef CAS PubMed.
- J. O. Woo, M. Misran, P. F. Lee and L. P. Tan, Sci. World J., 2014 DOI:10.1155/2014/205703.
- S. Y. Xie, L. Y. Zhu, Z. O. Dong, Y. Wang, X. F. Wang and W. Z. Zhou, Int J. Nanomed., 2011, 6, 547–555 CAS.
- D. Chirio, M. Gallarate, E. Peira, L. Battaglia, L. Serpe and M. Trotta, J. Microencapsulation, 2011, 28, 537–548 CrossRef CAS PubMed.
- L. Battaglia, M. Gallarate, R. Cavalli and M. Trotta, J. Microencapsulation, 2010, 27, 78–85 CrossRef CAS PubMed.
- N. Scholer, H. Hahn, R. H. Muller and O. Liesenfeld, Int. J. Pharm., 2002, 231, 167–176 CrossRef CAS.
- S. Jebors, A. Leydier, Q. Z. Wu, B. B. Ghera, M. Malbouyre and A. W. Coleman, J. Microencapsulation, 2010, 27, 561–571 CrossRef CAS PubMed.
- I. Montasser, P. Shahgaldian, F. Perret and A. W. Coleman, Int. J. Mol. Sci., 2013, 14, 21899–21942 CrossRef PubMed.
- J. Varshosaz, M. Tabbakhian and M. Y. Mohammadi, J. Liposome Res., 2010, 20, 286–296 CrossRef CAS PubMed.
- T. Helgason, T. S. Awad, K. Kristbergsson, E. A. Decker, D. J. McClements and J. Weiss, J. Agric. Food Chem., 2009, 57, 8033–8040 CrossRef CAS PubMed.
- M. Sznitowska, M. Gajewska, S. Janicki, A. Radwanska and G. Lukowski, Eur. J. Pharm. Biopharm., 2001, 52, 159–163 CrossRef CAS.
- J. Yi, T. I. Lam, W. Yokoyama, L. W. Cheng and F. Zhong, J. Agric. Food Chem., 2014, 62, 1096–1104 CrossRef CAS PubMed.
- C. M. Keck, A. Kovačević, R. H. Müller, S. Savić, G. Vuleta and J. Milić, Int. J. Pharm., 2014, 474, 33–41 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |

