Ultrasonic fabrication of TiO2/chitosan hybrid nanoporous microspheres with antimicrobial properties
Meifang Zhoua,
Bandar Babgib,
Shweta Guptaa,
Francesca Cavalieriac,
Yousef Alghamdib,
Mecit Aksub and
Muthupandian Ashokkumar*ab
aSchool of Chemistry, The University of Melbourne, Parkville, Melbourne, Victoria 3010, Australia. E-mail: masho@unimelb.edu.au; Tel: +61 3 93475180
bDepartment of Chemistry, Faculty of Science, King Abdulaziz University, P.O. Box 80203, Jeddah 21589, Saudi Arabia
cDipartimento di Scienze e Tecnologie Chimiche, Università![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) di Roma Tor Vergata, Roma, Italy
di Roma Tor Vergata, Roma, Italy
First published on 10th February 2015
Abstract
We report a sonochemical method for the fabrication of stable TiO2–chitosan hybrid microspheres possessing nanoporous structure and antimicrobial properties. The microspheres were characterized by evaluating their size, surface morphology, and ultrasound-triggered release of encapsulated materials. The size range of the microspheres was found to be in the range 2–20 μm. The antimicrobial activity of TiO2/chitosan microspheres was also evaluated. A possible mechanism for the formation of TiO2-encapsulated chitosan microspheres has been proposed. The methodology demonstrated here can be extended to fabricating nanoparticle or liquid delivery vectors and density controlled catalysts for applications in catalytic and biomedical areas.
Introduction
Microspheres with core–shell morphology are of significant interest due to their applications in the biomedical field, catalysis and the food industry. A variety of biopolymers have been used as shell materials. Chitosan has great potential in biomedical and pharmaceutical applications1–4 due to its favorable biological properties, such as biodegradability, biocompatibility, nontoxicity, high charge density, non-immunogenic and mucoadhesive properties. The free amino functional groups present within chitosan confer the biopolymer with a positive charge in acidic solutions and the ability to bind with many negatively charged materials and surfaces. The methods developed for fabricating chitosan microspheres include emulsification/solvent evaporation,5,6 spray drying,7,8 ionotropic gelation,9,10 and coacervation technique.11,12 However, poor mechanical strength of chitosan microspheres is still a challenging issue. Therefore, modification of the chitosan shell using metal or metal oxide to provide mechanical strength, stability and surface functionality is of great interest.Titanium dioxide (titania, TiO2) has attracted significant attention due to its promising applications in photocatalytic reactions, dye-sensitized solar cells, lithium ion batteries, gas sensors and drug delivery vehicles.13–20 Nanoparticles of titania are used in cosmetics and filters that exhibit strong germicidal properties. The loading of TiO2 into chitosan matrix could be a potential strategy for enhancing the mechanical property and antibacterial activity of chitosan microspheres.
Herein, we report on a one-step ultrasonic methodology for the fabrication of stable polysaccharide-based hybrid microspheres. In this approach, high intensity ultrasound is used to generate chitosan–TiO2 hybrid microspheres. Nanoporous TiO2 microspheres have been fabricated by extending the approach. TiO2 nanoparticles are used in photocatalytic applications. However, removal of the catalyst from aqueous solutions is difficult due to nanosized particles. Ultrasonically fabricated TiO2 hollow/porous microspheres would possess high surface area offering the advantage of nanoparticles and also could be easily filtered from an aqueous solution due to the larger size. The versatile procedure to produce TiO2-loaded chitosan microspheres or TiO2 hollow/porous microspheres is efficient, simple and fast and introduces a novel strategy to fabricate metal or metal oxide hollow/porous microspheres at low temperatures.
Experimental details
Materials
Titanium dioxide (P25) was from Degussa-Huls. Chitosan, yeast extract and Bacto tryptone were purchased from Sigma-Aldrich. Tetradecane (olefine free; >99%) was from Fluka. Milli-Q water was obtained from a Millipore system (18.2 MΩ cm at 25 °C).Fabrication methodology
Chitosan was dissolved in an aqueous acetic acid solution (pH 5) at 0.2% w/v. TiO2 (5 wt%) was added to chitosan solution and sonicated in an ultrasound bath for 15 min to generate a well dispersed TiO2 nanoparticles-containing chitosan solution. TiO2 is hydrophilic and could be easily dispersed in aqueous solutions. To prepare hydrophobic TiO2, TiO2 nanoparticles were dispersed in hexane using a sonication bath for 20 min and keep overnight. This was followed by the evaporation of hexane. The pre-treated TiO2 nanoparticles were dispersed in tetradecane (sonication bath for 20 min). Tetradecane containing dissolved nile red was chosen as a model organic liquid to demonstrate the versatility of the encapsulation procedure. TiO2-rich-chitosan-shelled microspheres were obtained by layering 50 μL tetradecane on the surface of chitosan/TiO2 mixture solution and sonicating at 160 W for 30 s by placing a horn microtip (3 mm in diameter; 20 kHz Branson sonifier) at the oil/solution interface. Chitosan microspheres containing TiO2-rich-core were fabricated by layering tetradecane/TiO2 mixture on the surface of chitosan solution followed by sonication under similar conditions as mentioned above. To the as-prepared sample, 1 M NaOH solution was added and the mixer was left overnight. The volume ratio of the as-prepared sample to NaOH solution was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and the solution pH was about 8–9. The samples were then washed 5 times with milli-Q water and finally stored in an aqueous solution at pH 7. In a typical washing procedure, the sonicated emulsion solution was left standing in a separation funnel for a few hours for the microspheres to float to the surface (due to relatively lower density). After draining the bottom section of the solution, milli-Q water was added to top “foamy” layer, shaken gently and left standing for a few hours. This procedure was repeated 5 times. TiO2 hollow/porous spheres were obtained after the removal of organic compounds by calcination at 450 °C for 10 h. The solution was dried in an oven at 110 °C prior to calcination.
10 and the solution pH was about 8–9. The samples were then washed 5 times with milli-Q water and finally stored in an aqueous solution at pH 7. In a typical washing procedure, the sonicated emulsion solution was left standing in a separation funnel for a few hours for the microspheres to float to the surface (due to relatively lower density). After draining the bottom section of the solution, milli-Q water was added to top “foamy” layer, shaken gently and left standing for a few hours. This procedure was repeated 5 times. TiO2 hollow/porous spheres were obtained after the removal of organic compounds by calcination at 450 °C for 10 h. The solution was dried in an oven at 110 °C prior to calcination.
Characterization
Optical microscopy (Olympus Model IX71) and scanning electron microscopy (FEI Quanta FEG ESEM) were used to examine the morphology and size of the microspheres. The average size and size distribution of microspheres were evaluated by measuring over 200 microspheres per system using optical microscopic and SEM images. High Resolution Transmission Electron Microscopy (HRTEM; FEI Tecnai F20) with selected area electron diffraction (SAED) was used to identify the structure of TiO2-loaded microspheres. The microspheres were embedded in LR-white resin and ultramicrotomed to 60 nm-thick sections. Fluorescence spectral measurements were carried out using a RF-5301 PC spectrophotometer (Shimadzu). Fluorescence optical microscopy (Olympus Model IX71) was used for detecting tetradecane or TiO2 encapsulated inside chitosan microspheres. Nile red was used as a fluorescence probe.The release of the encapsulated material was tested by ultrasonically breaking the microspheres using a high frequency (381 kHz) sonicator. 2 mL of microsphere sample was mixed with 4 mL hexane and sonicated in a vial by immersing the vial in water. The sonication led to the breakage of microspheres releasing the contents (nile red/tetradecane) into hexane. Fluorescence of nile red was monitored using a fluorescence spectrophotometer.
Antibacterial activity
Escherichia coli (DH 5 (alpha) strain) was grown in Luria Broth (LB) medium containing 5 g yeast extract, 10 g Bacto tryptone, and 5 g NaCl in 1000 mL distilled water. The pH of the medium was adjusted to ∼7. TiO2/chitosan hybrid microspheres were washed with milliQ water for 5 times and dried at 100 °C overnight. About 1 mL of the growth medium for E. coli with TiO2/chitosan samples (1 mg mL−1) was taken in separate falcon tubes to which 40 μL of E. coli (cell number = 6.78 × 104) was added. Control experiments were carried out with chitosan (1 mg mL−1) and TiO2 (mg mL−1). The mixture was incubated at 37 °C for 24 h. The antibacterial activity of the TiO2/chitosan microspheres for different incubation time was evaluated by measuring colony forming units (CFU) count method. Samples were taken after 2 h, 4 h, 6 h, 24 h for cell count measurements. The E. coli samples of different incubation were platted on agar plates for 12 h to evaluate the antibacterial activity of TiO2/chitosan microspheres. The CFU was determined by counting the colonies in agar plates.Results and discussion
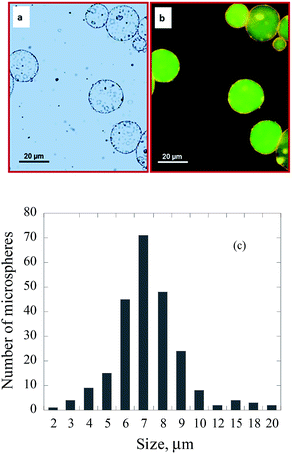
When a mixture of tetradecane and TiO2 nanoparticles was placed on the surface of an aqueous chitosan solution and sonicated as described in the experimental section, stable chitosan microspheres with tetradecane/TiO2 encapsulated in the core were formed (Fig. 1a). In order to confirm the encapsulation of tetradecane/TiO2, a fluorescent dye was added into the mixture of tetradecane/TiO2 prior to encapsulation. The fluorescence optical microscopic images (Fig. 1b) confirmed the encapsulation of nile red dye containing tetradecane/TiO2 within chitosan microspheres. The size of TiO2-loaded chitosan microspheres is in the range of 2–20 μm (Fig. 1c). TiO2 nanoparticles could also be loaded on chitosan shell when initially dispersed in the aqueous phase containing chitosan. The mechanism behind such processes will be discussed later.Earlier work on ultrasonic fabrication of proteinaceous microspheres21–23 suggests that stable microspheres could be formed when proteins containing cross-linkable functional groups are used. For example, the presence of thiol groups generated stable BSA microspheres.21 Recently, this concept has been supported by the observation that thiolated poly(methacrylic acid) formed stable microspheres when compared to unsubstituted poly(methacrylic acid).24
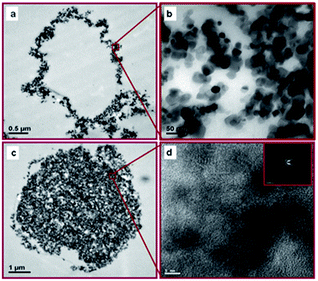
Chitosan is a non-sulfur-containing polysaccharide. However, various intermolecular forces such as hydrophobic interaction and H-bonding may play a role in the generation of stable chitosan microspheres.25 The structure and morphology of TiO2-loaded chitosan hybrid microspheres were examined by transmission electron microscopy (TEM) using ultramicrotomed sections of TiO2-loaded chitosan microspheres (Fig. 2).
Fig. 2 shows that TiO2 nanoparticles encapsulated in the shell of chitosan microspheres are around 20–25 nm in size. Fig. 2d shows that TiO2 crystalline nanoparticles are encapsulated in the core of chitosan microspheres. The negatively charged TiO2 nanoparticles bind with positively charged chitosan chains by electrostatic interactions to form a hybrid shell. TEM images also demonstrate that TiO2 loading can be controlled by choosing appropriate strategy. The dispersion of hydrophilic TiO2 nanoparticles in chitosan solution produced microspheres with TiO2 loaded on the chitosan shell (Fig. 2a), while the dispersion of hydrophobic TiO2 nanoparticles in tetradecane solution formed microspheres with a TiO2 core and a chitosan shell (Fig. 2c).
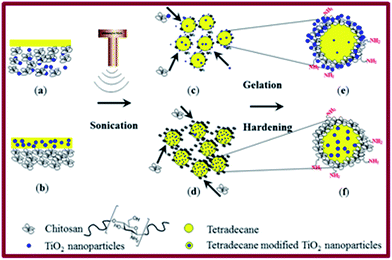
A possible mechanism for the formation of these completely different structures is schematically shown in Fig. 3. Sonication induces two processes: emulsification of an organic liquid (tetradecane without (a) or with TiO2 (b)) producing tetradecane droplets (c and d) in the aqueous medium and diffusion and adsorption of chitosan (or TiO2/chitosan) on the surface of tetradecane droplets (c and d) forming TiO2-loaded chitosan microspheres (e and f). Stable microspheres were generated by pH-induced hardening processes following sonication, which involved the dispersion of the microspheres in a basic (aqueous NaOH solution at pH 8–9) medium as described in the experimental section. Chitosan is soluble in acidic medium. Changing the solution pH to 8–9 leads to a lower solubility of chitosan in an aqueous medium that ultimately strengthens (hardens) the shell.
This versatile technique was also extended for fabricating hollow/porous TiO2 microspheres. Hollow/porous TiO2 microspheres were obtained by the evaporation of tetradecane encapsulated inside the TiO2 shell-loaded chitosan microspheres and removal of chitosan by calcination at 450 °C for 10 h. SEM images show that the size of porous and hollow TiO2 microspheres is about 5 μm (Fig. 4a), which are smaller than as-prepared TiO2 microspheres due to shrinkage after the removal of chitosan. The structure of the nanoparticle shelled hollow/porous TiO2 microspheres is well maintained after the heat treatment (Fig. 4b). The thickness of the shell after calcination is about 150–280 nm.
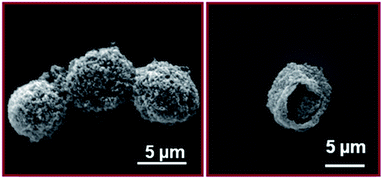 | ||
| Fig. 4 SEM images of tetradecane/TiO2-filled chitosan microspheres (a) and TiO2 hollow/porous microspheres after calcined at 450 °C for 10 h (b). | ||
In order to demonstrate the potential possibility of using TiO2-loaded chitosan microspheres for encapsulation and delivery applications, nile red dye was dissolved in tetradecane prior to encapsulation. Ultrasound-triggered release of the dye-loaded tetradecane was achieved and the results are shown in Fig. 5. In a typical experiment, a small amount of TiO2-loaded chitosan microspheres was dispersed in hexane and the fluorescence spectrum was recorded. Prior to sonication, nile red was encapsulated within TiO2–chitosan hybrid microspheres and hence fluorescence from nile red could not be observed. The dispersion was then sonicated at high frequency 381 kHz to break open the microspheres leading to the release of the dye into hexane. As can be seen from the fluorescence spectrum, the emission intensity increased substantially after the release of the dye.
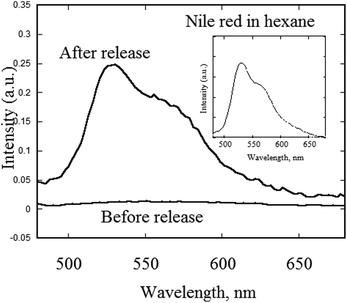 | ||
| Fig. 5 Fluorescence spectra of nile red dye-filled chitosan/TiO2 microspheres before and after ultrasound-induced release in hexane. | ||
The relative mechanical strengths of TiO2/chitosan hybrid microspheres and chitosan microspheres were determined by high frequency low-intensity ultrasound-induced release test. The sonication of microspheres at 381 kHz and 15 W for 5 min led to the release of tetradecane/nile red from both chitosan and TiO2/chitosan microspheres. By quantifying the amount of tetradecane/nile red released, the relative strengths of the microspheres could be determined. It has been observed that TiO2–chitosan hybrid microspheres are two times stronger than chitosan microspheres. Thus, loading of TiO2 onto chitosan to generate hybrid microspheres improved their mechanical strength.
The TiO2-loaded chitosan microspheres could be used in a number of applications. In order to demonstrate the versatility of this composite material, its antibacterial property has been demonstrated. The viability of E. coli stain in a culture medium containing the composite microspheres was evaluated as described in the experimental section. The digital photographs of E. coli cultured in the absence and presence of TiO2/chitosan composite microspheres are shown in Fig. 6.
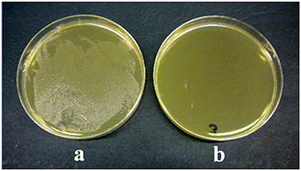 | ||
| Fig. 6 Photographs of E. coli (a) without and (b) with TiO2/chitosan hybrid microspheres after 6 hours. | ||
The data presented in Table 1 suggest that the presence of TiO2/chitosan hybrid microspheres deactivated more than 99% of E. coli after 6 h incubation (log reduction = 6.9) and in 24 h the growth was almost retarded (no cells were detected). When similar experiments were carried out with TiO2 and chitosan, no reduction in cells was observed (from 7.08 × 108 to 1.50 × 109) in both cases. In fact, a slight increase in cell number was observed up to 6 hours. Previous studies26,27 have shown that chitosan possesses antimicrobial property in acetic acid solutions (due to the existence of –NH3+ ions on chitosan). The absence of antimicrobial activity for chitosan in our study might be due to the use of undissolved chitosan in the growth solution.
| Incubation time (h) | Log reduction | ||
|---|---|---|---|
| TiO2 | Chitosan | TiO2–chitosan microspheres | |
| 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 1.3 |
| 4 | 0 | 0 | 2.2 |
| 6 | 0 | 0 | 6.9 |
A possible reason for the deactivation of E. coli by TiO2/chitosan composite microspheres could be due the photogenerated charge carriers from TiO2 interacting with the bacteria. Another possibility is the electrostatic interaction between the cationic amino groups of microspheres and the negatively charged bacterial cell membrane, which may have interfered with the stability of the cell structure leading to the deactivation of bacteria.
Conclusions
We have demonstrated a simple one-step sonochemical approach for fabricating TiO2–chitosan hybrid microspheres. Hydrophilic TiO2 nanoparticles can be encapsulated onto the shell of chitosan microspheres while hydrophobic TiO2 nanoparticles could be encapsulated into the core of chitosan microspheres. TiO2-loaded chitosan microspheres were found to show antimicrobial properties. Tetradecane encapsulated inside the chitosan microspheres could also be loaded with functional materials. For example, tetradecane can be replaced by dyes for the textile industry, antibacterial agents or drugs for medical applications or self-healing agents for paint industry. Furthermore, the ultrasonic approach can be extended for fabricating density controlled catalysts with hollow/porous features.Acknowledgements
This work was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, under grant no. (4-130-35-HiCi). The authors, therefore, acknowledge technical and financial support of KAU.Notes and references
- L. Illum, Pharm. Res., 1998, 15, 1326–1331 CrossRef CAS.
- M. Kumar, R. A. A. Muzzarelli, C. Muzzarelli, H. Sashiwa and A. Domb, Chem. Rev., 2004, 104, 6017–6084 CrossRef PubMed.
- I. Panos, N. Acosta and A. Heras, Curr. Drug Discovery Technol., 2008, 5, 333–341 CrossRef CAS.
- J. Varshosaz, Expert Opin. Drug Delivery, 2007, 4, 263–273 CrossRef CAS PubMed.
- I. Genita, P. Perugini, B. Conti and F. Pavanetto, Int. J. Pharm., 1997, 152, 237–246 CrossRef.
- P. Perugini, I. Genta, F. Pavanetto, B. Conti, S. Scalia and A. Baruffini, Int. J. Pharm., 2000, 196, 51–61 CrossRef CAS.
- J. Filipovic-Grcic, D. Voinovich, M. Moneghini, M. Becirevic-Lacan, L. Magarotto and I. Jalsenjak, Eur. J. Pharm. Sci., 2000, 9, 373–379 CrossRef CAS.
- P. He, S. S. Davis and L. Illum, Int. J. Pharm., 1999, 187, 53–65 CrossRef CAS.
- J. Berger, M. Reist, J. M. Mayer, O. Felt, N. A. Peppas and R. Gurny, Eur. J. Pharm. Biopharm., 2004, 57, 19–34 CrossRef CAS.
- A. Berthold, K. Cremer and J. Kreuter, J. Controlled Release, 1996, 39, 17–25 CrossRef CAS.
- M. A. Bayomi, S. A. Al-Suwayeh, A. M. El-Helw and A. F. Mesnad, Pharm. Acta Helv., 1998, 73, 187–192 CrossRef CAS.
- G. W. Vandenberg, C. Drolet, S. L. Scott and D. J. la Noue, J. Controlled Release, 2001, 77, 297–307 CrossRef CAS.
- U. Trudinger, G. Muller and K. K. Unger, J. Chromatogr., 1990, 535, 111–125 CrossRef.
- O. Carp, C. L. Huisman and A. Reller, Prog. Solid State Chem., 2004, 32, 33–177 CrossRef CAS PubMed.
- X. B. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- X. B. Chen, S. H. Shen, L. J. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- M. Grätzel, Nature, 2001, 414, 338–344 CrossRef PubMed.
- A. Fujishima, X. T. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef CAS PubMed.
- X. Y. Chen and W. Q. Luo, J. Nanosci. Nanotechnol., 2010, 10, 1482–1494 CrossRef CAS PubMed.
- M. Wagemaker, A. P. M. Kentgens and F. M. Mulder, Nature, 2002, 418, 397–399 CrossRef CAS PubMed.
- K. S. Suslick and M. W. Grinstaff, J. Am. Chem. Soc., 1990, 112, 7807–7809 CrossRef CAS.
- M. W. Grinstaff and K. S. Suslick, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 7708–7710 CrossRef CAS.
- F. Cavalieri, M. Colone, A. Stringaro, M. Tortora, A. Calcabrini, M. Zhou and M. Ashokkumar, Part. Part. Syst. Charact., 2013, 30, 695–705 CrossRef CAS.
- F. Cavalieri, M. Zhou, F. Caruso and M. Ashokkumar, Chem. Commun., 2011, 47, 4096–4098 RSC.
- N. Skirtenko, T. Tzanov, A. Gedanken and S. Rahimipour, Chem.–Eur. J., 2010, 16, 562–567 CrossRef CAS PubMed.
- X. F. Liu, Y. L. Guan, D. Z. Yang, Z. Li and K. D. Yao, J. Appl. Polym. Sci., 2001, 79, 1324–1335 CrossRef CAS.
- J. C. Fernandes, F. K. Tavaria, J. C. Soares, O. S. Ramos, M. J. Monterio, M. E. Pintado and F. X. Malcata, Food Microbiol., 2008, 25, 922–928 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |