Synthesis of magnetic graphene oxide-containing nanocomposite hydrogels for adsorption of crystal violet from aqueous solution†
Abstract
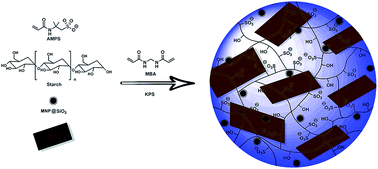
Magnetic nanocomposite hydrogels containing different amounts of graphene oxide were synthesized and characterized by FTIR, XRD, TGA, SEM, TEM, VSM and UV-vis spectroscopy. The prepared hydrogels were used as adsorbents for removal of a cationic dye, crystal violet, from water. The kinetics and isotherm of adsorption and the effect of different experimental conditions such as graphene oxide content, pH of the solution, contact time, adsorbent dosage and initial dye concentration on adsorption capacity were then investigated. Parameters related to kinetics and isotherm models were calculated and discussed. It was found that adsorption is well-described by pseudo-second-order kinetics and Langmuir isotherm models, respectively. The synthesized adsorbents showed high efficiency in removal of crystal violet and a very high adsorption capacity was obtained (769.23 mg g−1). No significant loss of removal efficiency was observed even after five cycles of adsorption.

 Please wait while we load your content...
Please wait while we load your content...