Unsaturated aldehydes: a novel route for the synthesis of pyridine and 3-picoline
Abstract
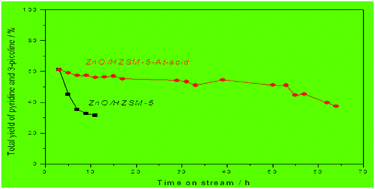
A novel reaction pathway was developed for the synthesis of pyridine and 3-picoline from the condensation of gas-phase acrolein dimethyl acetal or acrolein diethyl acetal and ammonia over various catalysts in a fixed-bed reactor. ZnO loaded on alkaline–acid sequentially-treated HZSM-5, namely ZnO/HZSM-5-At-acid, was prepared and employed in these reactions for the first time. 3-Picoline, without the generation of 4-picoline, was obtained from the condensation of acrolein dimethyl acetal and ammonia. The ZnO/HZSM-5-At-acid catalyst was proven to be the most promising catalyst relative to other catalysts in this study. The stability of the ZnO/HZSM-5-At-acid catalyst was remarkably higher than that of the ZnO/HZSM-5 catalyst. The catalysts were characterized using XRD, 27Al MAS NMR, XPS, UV-vis DRS, N2-physisorption, NH3-TPD and TG technologies and the results revealed that the pore structure, acidity and location of ZnO had great influence on the total yield of pyridine and 3-picoline, and the catalyst stability.

 Please wait while we load your content...
Please wait while we load your content...