An Fe3O4@(C–MnO2) core–double-shell composite as a high-performance anode material for lithium ion batteries
Yanqing Fu,
Xianyou Wang*,
Hao Wang,
Youwei Zhang,
Xiukang Yang and
Hongbo Shu*
Key Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education, Hunan Province Key Laboratory of Electrochemical Energy Storage and Conversion, School of Chemistry, Xiangtan University, Hunan, Xiangtan 411105, China. E-mail: wxianyou@yahoo.com; hongboshuxtu@gmail.com; Fax: +86 731 58292061; Tel: +86 731 58292060
First published on 14th January 2015
Abstract
An Fe3O4@(C–MnO2) composite with a cube-like core–double-shell structure has been successfully designed and prepared by a combination of the hydrothermal method and a layer-by-layer (LBL) self-assembly technique. This novel hybrid composite was characterized by X-ray powder diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray (EDX) spectroscopy and electrochemical tests. It has been found that this material has a cube-like morphology with a core–double-shell structure. Compared with the bare α-Fe2O3 and Fe3O4–C materials, the as-prepared composite has a significantly enhanced electrochemical performance, with a high capacity, good rate capability, and excellent cycling stability as an anode material for lithium ion batteries (LIBs). At a current density of 100 mA g−1, the as-obtained Fe3O4@(C–MnO2) composite electrode delivers a reversible capacity exceeding 1000 mA h g−1 and retains 979 mA h g−1 after 150 cycles. In contrast, the discharge capacities of the bare α-Fe2O3 and Fe3O4–C show only 111 mA h g−1 and 282 mA h g−1 at a current density of 100 mA g−1 after 150 cycles, respectively. This improved electrochemical performance can be attributed to the high theoretical capacity and larger specific surface area of the MnO2 layer, as well as the high electrical conductivity of the carbon layer, which acts as both the linker and the stabilizer between Fe3O4 and MnO2.
1. Introduction
In recent years, the search for high-performance electrode materials with the capability of storing and delivering energy efficiently for lithium ion batteries (LIBs) has generated a great deal of interest.1–4 In terms of anode materials, traditional graphite is widely used in commercial LIBs because of its low cost and long cycle life. However, its low theoretical capacity (372 mA h g−1) limits its widespread application in high-performance LIBs. Compared with carbon-based materials, transition metal oxides (TMOs), with a novel conversion mechanism that could offer a high theoretical specific capacity (∼1000 mA h g−1), are supposed to be one of the most promising anode materials. Examples include α-Fe2O3,5 MnO2,6 Co3O4,7 NiO,8 Fe3O4,9 and so forth. Among all TMOs, iron oxide has attracted much attention owing to its low toxicity, plentiful raw materials, high corrosion resistance, improved safety and low processing cost.10–12 Nevertheless, its poor cycling stability and low rate capability, caused by the large specific volume changes during the charge–discharge cycling process, and the kinetic limitations of its intrinsic nature, still strongly restrict its large-scale practical application.To solve this problem, surface modification has been proven effective. It has been reported that carbon coating is the most widely-used technique to increase the electronic conductivity of electrode materials and maintain the integrity of particles.10,12,13 Zhang et al.14 synthesized an Fe3O4/C nanospindle as an anode material by the hydrothermal treatment of α-Fe2O3 precursors with glucose. It showed a high reversible capacity of 745 mA h g−1 at a current density of 185 mA g−1. In addition, Xiong et al.15 fabricated porous Fe3O4/C core–double-shell nanorods through the partial reduction of porous α-Fe2O3 particles with carbon coating and obtained a specific capacity of 762 mA h g−1 at 92.4 mA g−1 after 50 cycles. Liu et al.16 prepared Fe3O4/C composites with a specific capacity of 488 mA h g−1 at 60 mA g−1. However, as the buffer for mitigating the volume change during cycling, carbon on one side of the active materials didn't allow them to operate at their maximum capacity. To resolve this drawback, assembly of different oxides into a hierarchical composite is a good solution and a research hotspot, and could take full advantage of the different components for better performance in LIBs.17,18 Very recently, another environmentally-friendly and resourceful compound manganese dioxide (MnO2), with a high theoretical capacity and relatively-low electrochemical motivation force, has attracted attention due to its wide applications in catalysis and energy storage.19–21 Notably, it has been reported that incorporating nanostructured MnO2 in a conductive layer-by-layer (LBL) deposition can produce anode materials with high reversible capacities for LIBs.22,23
Herein, an Fe3O4@(C–MnO2) core–double-shell composite with a cube-like morphology was successfully designed and synthesized by a mild hydrothermal reaction and the LBL technique. Specifically, Fe3O4–C particles were first prepared via partial reduction of α-Fe2O3 cubes with carbon coating, and then MnO2 nanomaterials were deposited on the surface of the Fe3O4–C by utilizing the in situ chemical redox reaction between the carbon layer and KMnO4 to form the Fe3O4@(C–MnO2) composite, as illustrated in Scheme 1. To the best of our knowledge, such a novel core–double-shell Fe3O4@(C–MnO2) composite, which is found to show improved electrochemical properties including a high specific capacity and improved cycling performance, has rarely been reported. Moreover, the physicochemical and electrochemical properties of the as-prepared Fe3O4@(C–MnO2) composite are studied in detail.
 | ||
| Scheme 1 Schematic illustration of the synthetic process of the cube-like Fe3O4@(C–MnO2) core–double-shell composite. | ||
2. Experimental
2.1 Synthesis of α-Fe2O3 cubes
All the reactants below were of analytical grade and used without further purification. In a typical synthesis, 30 mL deionized water and 30 mL absolute ethanol were mixed together, then 1.3 g (4.8 mmol) FeCl3·6H2O and 0.3 g (5.0 mmol) urea were added to the solution. After stirring to form a homogeneous solution, the mixture was transferred into a 100 mL Teflon autoclave and then reacted at 180 °C for 6 h in an oven. After cooling down to room temperature, the precipitate was collected by centrifugation and then washed with deionized water and absolute ethanol several times, followed by drying at 70 °C for 12 h in an oven. Finally, the desiccated sample was heated in a quartz tube to 400 °C in air at a rate of 5 °C min−1 and kept at this temperature for 3 h to obtain reddish-brown α-Fe2O3 submicron cubes.2.2 Synthesis of Fe3O4–C particles
0.6 g of as-prepared hematite particles was mixed with 2.5 g of solid citric acid in ethanol by grinding gently, the powder was then obtained after the evaporation of ethanol. Finally, the desiccated sample was carbonized at 600 °C for 4 h in a N2 atmosphere at a rate of 5 °C min−1, to obtain black Fe3O4–C particles.2.3 Synthesis of Fe3O4@(C–MnO2) particles
0.3 g of the as-prepared carbon-coated Fe3O4 particles was dispersed in 75 mL of 0.03 M KMnO4 aqueous solution under high-power ultrasonication for 1 h. Then, the suspension was transferred into a 100 mL Teflon autoclave and kept at 180 °C for 6 h in an oven. After cooling down to room temperature, the chocolate-colored product was collected by centrifugation and washed with deionized water and absolute ethanol several times, followed by drying at 120 °C for 12 h in an oven.2.4 Physical characterization
The phase purity and structure of the as-synthesized samples were characterized by X-ray powder diffraction (XRD) using a Rigaku D/MAX-2500 powder diffractometer with a graphite monochromator and Cu Kα radiation (λ = 0.15418 nm), operated at a scan rate of 2° min−1 in the 2θ range of 10–80°, with a step size of 0.01°. Scanning electron microscopy (SEM) images were collected using a JEOL JSM-6610 scanning electron microscope. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) observations, as well as energy dispersive X-ray (EDX) spectroscopy analyses were obtained using a JEOL JEM-2100F transmission electron microscope at an acceleration voltage of 200 kV. The specific surface area and pore size distribution curves of the samples were determined from N2 adsorption–desorption isotherms measured at 77 K (JW-BK112). The chemical compositions of the as-synthesized samples were detected using atomic absorption spectroscopy (AAS, Vario 6 Analytik Jena AG, Jena). The thermogravimetric analysis (TGA) was carried out on a TGA Q50 V20.8 Build 34.2.5 Electrochemical measurements
Electrochemical tests of the as-prepared samples were carried out using two-electrode button-type cells assembled in an argon-filled glove box, where the water and oxygen concentrations were kept at less than 5 ppm. The working electrodes were fabricated by mixing 70 wt% of the active materials, 20 wt% acetylene black and 10 wt% polymer binder (polyvinylidene fluoride, PVDF), which were then pasted on a copper foil, followed by drying under vacuum at 110 °C for 10 h. A lithium disc served as both the counter electrode and the reference electrode. 1 M LiPF6 in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) was used as an electrolyte and the separator was a Celgard 2400. The galvanostatic charge–discharge measurements were performed using a Neware battery tester BTS-XWJ-6.44S-00052 (Neware, Shenzhen, China) at different current densities, with a cut-off voltage window of 0.01–3.0 V. The calculation of the specific capacity was based on the overall mass of the composite synthesized. Cyclic voltammetry (CV) tests were carried out on a VersaSTAT3 electrochemical workstation (Princeton, America) at a scan rate of 0.1 mV s−1 with the potential interval 0.01–3.0 V (vs. Li+/Li). Electrochemical impedance spectroscopy (EIS) was also performed using a VersaSTAT3 electrochemical workstation by applying an ac amplitude of 5 mV over the frequency range of 105 to 0.01 Hz. All the electrochemical measurements were performed at room temperature.
v) was used as an electrolyte and the separator was a Celgard 2400. The galvanostatic charge–discharge measurements were performed using a Neware battery tester BTS-XWJ-6.44S-00052 (Neware, Shenzhen, China) at different current densities, with a cut-off voltage window of 0.01–3.0 V. The calculation of the specific capacity was based on the overall mass of the composite synthesized. Cyclic voltammetry (CV) tests were carried out on a VersaSTAT3 electrochemical workstation (Princeton, America) at a scan rate of 0.1 mV s−1 with the potential interval 0.01–3.0 V (vs. Li+/Li). Electrochemical impedance spectroscopy (EIS) was also performed using a VersaSTAT3 electrochemical workstation by applying an ac amplitude of 5 mV over the frequency range of 105 to 0.01 Hz. All the electrochemical measurements were performed at room temperature.
3. Results and discussion
3.1 Structural and morphological analysis
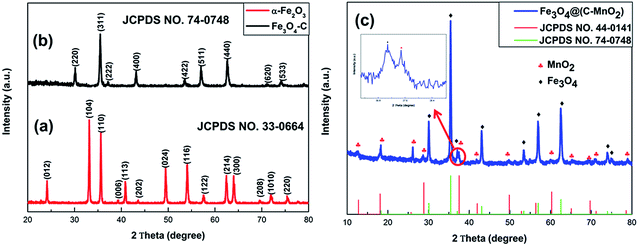
X-ray diffraction (XRD) analysis was used to determine the phase structures of the samples. Fig. 1a is the XRD pattern of the as-prepared α-Fe2O3, in which all the diffraction peaks are in good agreement with the standard hematite (α-Fe2O3) crystal structure (JCPDS no. 33-0664), indicating that a pure and highly-crystalline product has been obtained. The XRD pattern of Fe3O4–C, after coating with a carbon layer, is shown in Fig. 1b. All peaks indicated by Miller indices in the pattern can be well indexed to the face-centered Fe3O4 (JCPDS no. 74-0748), which demonstrates that the pure α-Fe2O3 was converted to Fe3O4.11,14 Besides, no other diffraction peaks can be obviously observed, suggesting an amorphous carbon. The diffraction pattern of the MnO2-modified Fe3O4–C particles (Fig. 1c) shows more peaks in different positions from those of Fe3O4–C, which are consistent with the peaks seen in the pattern of MnO2 (JCPDS no. 44-0141), indicating that MnO2 is present in the Fe3O4@(C–MnO2) sample.Typical scanning electron microscopy (SEM) images were obtained in order to understand the morphologies and microstructures of the as-obtained samples, as shown in Fig. 2. It is quite clear that the structure of the α-Fe2O3 precursor is cubic with a size of 400–800 nm, and the color is reddish-brown (inset in Fig. 2a). After sintering the mixture of α-Fe2O3 particles with citric acid, the morphology of Fe3O4–C, as displayed in Fig. 2b, was perfectly retained as a cube-like structure, without noticeable variation compared with that of the α-Fe2O3 precursor, except that the outer surface became coarse and the color changed to black (inset in Fig. 2b), indicating that the carbon had coated the surface of the Fe3O4 particles. After a mild hydrothermal reaction between KMnO4 and the Fe3O4–C cubes, Fe3O4@(C–MnO2) still retained the cubic morphology, as shown in Fig. 2c, but revealed a rougher surface than the Fe3O4–C. Moreover, the color of the sample changed to a chocolate color (inset in Fig. 2c). This indicates that MnO2 is probably introduced.
To get further insight into the morphology and structure of the samples, transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) observations were carried out. Fig. 3a and b show that the morphology of the precursor is cubic and the lattice spacing of 0.37 nm can be assigned to the (012) plane of α-Fe2O3, consistent with SEM and XRD, respectively. The TEM image in Fig. 3d shows that the cube-like α-Fe2O3 is coated with carbon. Further information can be learned from Fig. 3e. It is quite clear that the amorphous carbon layer has coated the surface, and the lattice fringes with spacings of 0.48 nm and 0.25 nm are attributed to the (111) and (311) planes of the face-centered Fe3O4, respectively, indicating that α-Fe2O3 was converted to Fe3O4 after calcining. This is consistent with the XRD pattern of Fe3O4–C. The Fe3O4–C particles can be encapsulated by the amorphous MnO2 layer that is produced by the chemical redox reaction between the carbon layer and KMnO4, based on the following reaction.
| 4KMnO4 + 3C + H2O → 4MnO2 + 2KHCO3 + K2CO3 | (1) |
 | ||
| Fig. 3 TEM images, HRTEM images and EDX spectra of (a–c) α-Fe2O3, (d–f) Fe3O4–C and (g–i) Fe3O4@(C–MnO2). | ||
Thus, a rough surface with some needle-like and flocculus-like nanomaterials is observed, as shown in Fig. 3g, resulting in a higher surface area. The inset of Fig. 3g is from the partial area of the TEM image (the red circle in Fig. 3g), which is magnified to confirm the presence of the needle-like MnO2 layer deposited on the surface of the carbon layer. From this, the carbon and MnO2 layers are clearly seen. Furthermore, from the HRTEM, as displayed in Fig. 3h, the lattice fringes with interplanar spacings of 0.21 nm, 0.19 nm and 0.24 nm are in good accordance with the (301), (510) and (211) planes of MnO2 (JCPDS no. 44-0141), respectively. This result is consistent with the XRD data. Obviously, the middle layer is amorphous carbon in Fig. 3h. On the other side of the carbon, the lattice fringe of 0.29 nm is observed, corresponding to the planes of the face-centered Fe3O4. From Fig. 3g and h, it can be seen that the Fe3O4@(C–MnO2) core–double-shell composite with a cube-like morphology was successfully prepared.
Energy-dispersive X-ray (EDX) analysis was used to further verify the elements present in the samples. Except for Cu (the Cu peaks in all the spectra come from the copper substrates), it can be observed from Fig. 3f and i that Fe, O and C exist in both the Fe3O4–C and Fe3O4@(C–MnO2) samples, indicating the presence of carbon. It should be noted that there is also C present in Fig. 3c, but the C peak intensity is lower than in the Fe3O4–C and Fe3O4@(C–MnO2) samples, which may be attributed to the use of the TEM grid (containing carbon) in the test. Particularly, Fig. 3i reveals that the final product is composed of Fe, O, C and Mn. Remarkably, the weak peaks located at 3.5–4.0 keV are likely to be from the KHCO3 and/or K2CO3 formed during the redox deposition of MnO2.23
Nitrogen adsorption–desorption measurements were carried out at 77 K to study the textural characteristics of these three samples. Fig. 4c shows the nitrogen sorption data of Fe3O4@(C–MnO2), revealing a Brunauer–Emmett–Teller (BET) surface area of 32.50 m2 g−1, which is much higher than those of α-Fe2O3 (8.66 m2 g−1, Fig. 4a) and Fe3O4–C (10.89 m2 g−1, Fig. 4b). The pore size distributions (insets) of all three samples are derived from the adsorption branches of the isotherms, based on the Barrett–Joyner–Halenda (BJH) model. They are mainly in the range of 1.5–5 nm, and the mean pore diameters are 2.87, 2.86 and 2.65 nm for α-Fe2O3, Fe3O4-C and Fe3O4@(C–MnO2), respectively.
Thermogravimetric analysis (TGA) was carried out in air at a heating rate of 5 °C min−1 to analyze the carbon content in Fe3O4@(C–MnO2). In Fig. 4d, because of removal of the weakly-adsorbed water and other small volatile molecules, the TGA curve displays a first weight loss from 50 °C to 200 °C. Then a following weight gain (∼2.0 wt%) corresponds to the transformation of Fe3O4 to Fe2O3. The second weight loss at a higher temperature can be mainly attributed to the evaporation and subsequent decomposition of the amorphous carbon layer. Therefore, there is ∼12.8 wt% carbon in Fe3O4@(C–MnO2) from the TGA. Furthermore, according to the atomic absorption spectroscopy (AAS), the Fe3O4@(C–MnO2) composite is composed of 61.5 wt% Fe3O4 and 26.0 wt% MnO2. As a result, the theoretical capacity of Fe3O4@(C–MnO2) is about 935.1 mA h g−1 (935.1 mA h g−1 = (924 mA h g−1 × 61.5 wt%) + (372 mA h g−1 × 12.5 wt%) + (1232 mA h g−1 × 26.0 wt%)).
3.2 Electrochemical analysis
Fig. 5a, c and e show the typical cyclic voltammetric (CV) curves for the α-Fe2O3, Fe3O4–C and Fe3O4–C@MnO2 electrodes between 0.01 and 3 V at a scan rate of 0.1 mV s−1. With regard to Fig. 5a and c, the cathodic current peaks positioned at 0.58 V and 0.51 V in the first cycle can respectively be ascribed to a reversible conversion reaction of α-Fe2O3 and Fe3O4 with the metallic lithium to form lithia (Li2O) and metallic iron (Fe0), and the electrolyte decomposition to form solid electrolyte interphase (SEI) films.24,25 In addition, in the anodic processes, two peaks are present at 1.65 V and 1.66 V, corresponding to the reversible oxidation process of Fe0 to Fe3+.26 Apparently, the peak intensities of the two samples drop dramatically in the second cycles, indicating the occurrence of some irreversible processes in the first cycling process, which can be attributed to the formation of an SEI film and electrolyte decomposition. Furthermore, the intensities of the 30th and 50th curves are obviously reduced, thus resulting in an inferior capacity. For the Fe3O4@(C–MnO2) electrode (Fig. 5e), in the first cycle, the fairly sharp cathodic current peak is located at about 0.20 V, and the other cathodic peak is positioned at 0.60 V, which can be attributed to the reversible reduction of MnO2 and Fe3O4 with lithium in the electrode, respectively. Meanwhile the anodic peaks seen at 1.21 V and 1.60 V can separately be assigned to the electrochemical oxidation reaction for MnO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 and Fe3O4. Due to the polarization of active materials and the formation of an SEI layer in the first cycle, all the cathodic and anodic peaks are shifted slightly toward to a more positive potential, and the peak intensities decrease during the second cycle. It is worth noting that the CV curves overlap well during the 30th and 50th cycles (Fig. 5e). This result means that the electrochemical reaction is highly reversible.
27 and Fe3O4. Due to the polarization of active materials and the formation of an SEI layer in the first cycle, all the cathodic and anodic peaks are shifted slightly toward to a more positive potential, and the peak intensities decrease during the second cycle. It is worth noting that the CV curves overlap well during the 30th and 50th cycles (Fig. 5e). This result means that the electrochemical reaction is highly reversible.
The charge–discharge cycles for the prepared samples were tested within the cutoff voltage window of 0.01–3.0 V at a current density of 100 mA g−1 in coin-type lithium half cells, and the corresponding profiles are shown in Fig. 5b, d and f. As shown in Fig. 5b, the first discharge curve of α-Fe2O3 has an obvious potential plateau at about 0.8 V, which is similar to that reported for α-Fe2O3.28,29 It is evident that the discharge process of Fe3O4–C, with an accordant voltage plateau of about 0.75 V, manifests typical anode voltage trends of Fe3O4.30 In the case of the Fe3O4@(C–MnO2), flat discharge plateaus at around 0.8 V and 0.45 V are observed in the first discharge cycle (Fig. 5f), correlating with the reduction processes for Fe3O4 and MnO2. The first specific discharge capacities for α-Fe2O3, Fe3O4–C and Fe3O4@(C–MnO2) are 1018, 1336 and 1786 mA h g−1, respectively. However, the discharge capacities for α-Fe2O3 and Fe3O4–C fade faster than that for Fe3O4@(C–MnO2) during the subsequent cycles, with only 385 and 782 mA h g−1 for the formers but 1300 mA h g−1 for Fe3O4@(C–MnO2) in the second cycles. Thus, the bare α-Fe2O3 and Fe3O4–C show remarkable irreversible capacity loss, arising from the relatively-large volume change during the lithiation/delithiation process and the decomposition of solvent in the electrolyte forming a SEI.31,32 Further, the discharge capacities of the α-Fe2O3 and Fe3O4–C drop rapidly to 118 and 294 mA h g−1, respectively. In contrast, that of Fe3O4@(C–MnO2) reduces slightly and still remains at a high capacity of 979 mA h g−1 for up to 150 cycles. Accordingly, the capacity and cycling stability of Fe3O4@(C–MnO2) are significantly improved.
 | ||
| Fig. 5 CV curves and charge–discharge profiles of (a and b) α-Fe2O3, (c and d) Fe3O4–C and (e and f) Fe3O4@(C–MnO2) electrodes between 0.01 and 3 V at a scan rate of 0.1 mV s−1. | ||
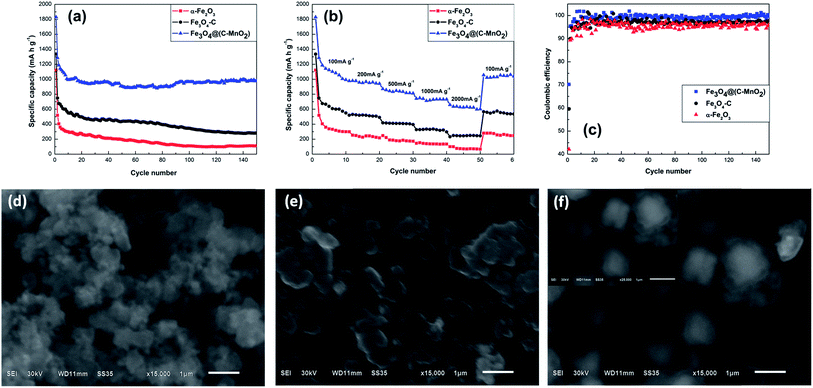
Fig. 6a compares the cycling performances of the three samples at a current density of 100 mA g−1 in the voltage range of 0.01–3 V. Since the carbon layer can provide mechanical protection and stabilize the SEI layer at a lower potential, the Fe3O4–C exhibits a better cycling performance than the pure α-Fe2O3, and the discharge capacity of Fe3O4–C stabilizes at ∼435 mA h g−1, whereas that of α-Fe2O3 is only stable at ∼141 mA h g−1. Moreover, owing to the larger specific surface area and high theoretical capacity of MnO2 located on the surface of the Fe3O4–C, the specific discharge capacity of the Fe3O4@(C–MnO2) can be greatly improved to ∼970 mA h g−1, which is over twofold that of Fe3O4–C. Coulombic efficiency (CE) is an important parameter to evaluate electrochemical performance.33,34 The CEs of the three samples are compared in Fig. 6c. It can be seen that the initial CE of the Fe3O4@(C–MnO2) is ∼70%, which is significantly higher than that of α-Fe2O3 (∼42%) and Fe3O4–C (∼60%). After several cycles, the CE of Fe3O4@(C–MnO2) increased to about 99%, however, this was only ∼96% and ∼97% for α-Fe2O3 and Fe3O4–C, respectively. Therefore, it can be concluded that the combination of the carbon coating as well as the MnO2 layer makes an obvious contribution to the higher cycling performance, capacity and higher CE for the Fe3O4@(C–MnO2). In particular, the high surface area and high theoretical capacity of MnO2 could contribute to the excellent capacity of Fe3O4@(C–MnO2); besides, the loose MnO2 layer could provide buffering space to accommodate the huge volume change of the active materials and prevent the aggregation of particles during cycles, thus improving the cycling performance of the composite. Interestingly, from the 80th cycle onward, the capacity of the Fe3O4@(C–MnO2) increased gradually to a value as high as 979 mA h g−1 until 150 cycles. This phenomenon may probably be attributed to the reversible formation of organic polymeric/gel-like films by decomposition at low potentials.35,36
To investigate the effect of MnO2 on the structure of Fe3O4@(C–MnO2), SEM images were taken after 150 cycles at 100 mA g−1 for all three samples. As shown in Fig. 6f, although the size of the particles has increased a little after 150 cycles, the structure of Fe3O4@(C–MnO2) is retained well without agglomeration, which can be ascribed to the fact that the MnO2 layer can buffer volume expansion and alleviate the structure damage during cycling. On the contrary, as for α-Fe2O3 (Fig. 6d), without any protecting layers, the sample looks seriously aggregated and pulverized. In the meantime, Fig. 6e shows that the Fe3O4–C particles have not collapsed as severely as the α-Fe2O3, due to the protection of the carbon layer. In conclusion, the MnO2 layer can preserve the structural integrity of Fe3O4@(C–MnO2) well during long-term charge–discharge cycles.
Rate capability is also a characteristic of great significance for high-performance LIBs. Fig. 6b illustrates the rate performance of α-Fe2O3, Fe3O4–C and Fe3O4@(C–MnO2) electrodes at various current densities from 100 to 2000 mA g−1, so as to examine the effects of the carbon and MnO2 layers on rate capability. On account of the superior electronic conductivity of carbon, the Fe3O4–C exhibits a better rate capability than the bare α-Fe2O3. The discharge capacities of ∼650, ∼410 and ∼250 mA h g−1 for Fe3O4–C are much higher than ∼400, ∼200 and ∼75 mA h g−1 for α-Fe2O3 under the current densities of 100, 500 and 2000 mA g−1, respectively. In the case of Fe3O4@(C–MnO2), at a low current density of 100 mA g−1, the discharge capacity is as high as ∼1100 mA h g−1, especially, even as the current densities increase to 500 and 2000 mA g−1, it still delivers discharge capacities of ∼860 and ∼630 mA h g−1, respectively. Compared with Fe3O4–C, it can be unambiguously seen that Fe3O4@(C–MnO2) possesses a remarkably high capacity and improved rate performance. When the current density finally returns to its initial value of 100 mA g−1, the discharge capacity of ∼1040 mA h g−1 can still be observed for the Fe3O4@(C–MnO2) sample, but only ∼545 and ∼260 mA h g−1 are observed for Fe3O4–C and α-Fe2O3, respectively, which further verifies the merits of this core–double-shell structure. Consequently, it is worth noting that the superior rate performance of Fe3O4@(C–MnO2) is most likely owing not only to the high electronic conductivity of the carbon layer but also to the high-capacity combination of MnO2 and Fe3O4.
To further understand the superior electrochemical performance of Fe3O4@(C–MnO2) as an anode material, electrochemical impedance spectroscopy (EIS) measurements of the three electrodes were carried out at around 2.5 V (room temperature) on cells comprising the three samples as the working electrodes versus Li before the discharge–charge cycles. As shown in Fig. 7a, the Nyquist plots of the three electrodes are similar to each other, with a semicircle at high–medium frequency and an inclined straight line at low frequency. It is well known that the high-frequency semicircle is related to the ohmic resistance of the cell from Z′ axis interception and attributed to the contact resistance, polarization resistance, charge transfer resistance and corresponding capacitances interception. The oblique line in the low frequency range indicates the Warburg impedance (Ws), which is associated with lithium ion diffusion through electrodes. The equivalent circuit model (in Fig. 7b) is used to fit the EIS data, where Rs is known as the ohmic resistance, and Rct is the charge transfer resistance. Nyquist plots are fitted as the magenta curves (in Fig. 7a) and the fitted impedance data are listed in Table 1. As shown in Fig. 7a, the fitting patterns are in good agreement with the experimental EIS data. It can be seen from Table 1 that the Rs data of three samples show slight differences. The Rs of Fe3O4@(C–MnO2) (5.116 Ω) is slightly higher than that of the others, owing to the low electronic conductivity of MnO2. In addition, the Rct value of Fe3O4@(C–MnO2) (111.8 Ω) is less than those of α-Fe2O3 (373.1 Ω) and Fe3O4–C (231.1 Ω), indicating the lowest charge-transfer resistance in the Fe3O4@(C–MnO2) electrode. These results suggest that the Fe3O4@(C–MnO2) electrode has the lowest activation energy for Li+ diffusion and undergoes a fast Faradaic reaction, confirming the significantly enhanced cycling performance and electrochemical dynamic behavior of the Fe3O4@(C–MnO2) anode in comparison to the other two electrodes.
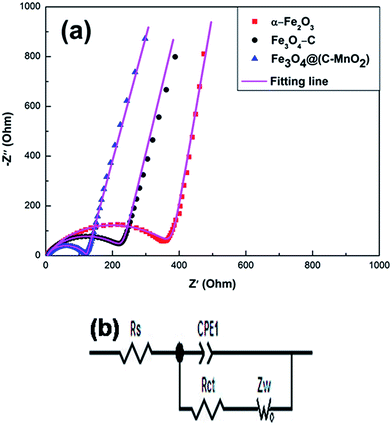 | ||
| Fig. 7 (a) The electrochemical impedance spectra (EIS) of the α-Fe2O3, Fe3O4–C and Fe3O4@(C–MnO2) electrodes. (b) The equivalent circuit used for fitting the experimental EIS data. | ||
| Samples | α-Fe2O3 | Fe3O4–C | Fe3O4@(C–MnO2) |
|---|---|---|---|
| Rs (Ω) | 5.090 | 5.045 | 5.116 |
| Rct (Ω) | 373.1 | 231.1 | 111.8 |
4. Conclusions
In summary, we have successfully synthesized the unique Fe3O4@(C–MnO2) core–double-shell composite with a cube-like morphology as an anode material for LIBs by the hydrothermal method and LBL technique. Fe3O4–C particles were prepared via partial reduction of α-Fe2O3 cubes with carbon coating in a calcination process; the Fe3O4@(C–MnO2) core–double-shell composite was further formed by a mild hydrothermal redox reaction between KMnO4 and Fe3O4–C. The Fe3O4@(C–MnO2) composite exhibits a markedly improved electrochemical performance, including a higher discharge capacity exceeding 1000 mA h g−1 at a current density of 100 mA g−1, a superior rate capability with the discharge capacity of ∼630 mA h g−1 even as the current density increases to 2000 mA g−1, and excellent cycling stability with a capacity of 979 mA h g−1 after 150 cycles at a current density of 100 mA g−1. These outstanding performances can mainly be attributed to the high theoretical capacity and larger specific surface area of MnO2. In addition, the MnO2 nanomaterials deposited on the surface of Fe3O4–C materials are of benefit to further accommodate the large volume change and alleviate structure damage during battery cycling to some extent. Therefore, this novel Fe3O4@(C–MnO2) core–double-shell architecture with a cube-like morphology could be extended to other active TMOs, thus providing an innovative strategy for designing new anode materials for next-generation LIBs.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant no. 51072173, 51272221, 51302239 and 21203161), Specialized Research Fund for the Doctoral Program of Higher Education (Grant no. 20134301130001), and the Natural Science Foundation of Hunan Province, China (Grant no. 13JJ4051).References
- J. Jiang, Y. Li, J. Liu, X. Huang, C. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166 CrossRef CAS PubMed.
- L. Ji, Z. Lin, M. Alcoutlabi and X. Zhao, Energy Environ. Sci., 2011, 4, 2682 CAS.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930 CrossRef CAS PubMed.
- S. Mitra, P. Poizot, A. Finke and J.-M. Tarascon, Adv. Funct. Mater., 2006, 16, 2281 CrossRef CAS.
- J. Zhu, Z. Yin, D. Yang, T. Sun, H. Yu, H. E. Hoster, H. H. Hng, H. Zhang and Q. Yan, Energy Environ. Sci., 2013, 6, 987 CAS.
- H. Wang, J. Liu, X. Wang, C. Wu, Q. Zhao, Q. Fu, X. Yang and H. Shu, RSC Adv., 2014, 4, 22241 RSC.
- D. Su, S. Dou and G. Wang, Nano Res., 2014, 7, 794 CrossRef CAS.
- Y. Zhu, H. Guo, Y. Wu, C. Cao, S. Tao and Z. Wu, J. Mater. Chem. A, 2014, 2, 7904 CAS.
- Y. Chen, B. Song, X. Tang, L. Lu and J. Xue, J. Mater. Chem., 2012, 22, 5006 RSC.
- T. Muraliganth, A. V. Murugan and A. Manthiram, Chem. Commun., 2009, 7360 RSC.
- L. Lang and Z. Xu, ACS Appl. Mater. Interfaces, 2013, 5, 1698 CAS.
- L. Bo, H. Cao, J. Shao and M. Qu, Chem. Commun., 2011, 47, 10374 RSC.
- L. J. Fu, H. Liu, H. P. Zhang, C. Li, T. Zhang, Y. P. Wu, R. Holze and H. Q. Wu, Electrochem. Commun., 2006, 8, 1 CrossRef CAS PubMed.
- W. M. Zhang, X. L. Wu, J. S. Hu, Y. G. Guo and L. J. Wang, Adv. Funct. Mater., 2008, 18, 3941 CrossRef CAS.
- Q. Q. Xiong, Y. Lu, X. L. Wang, C. D. Gu, Y. Q. Qiao and J. P. Tu, J. Alloys Compd., 2012, 536, 219 CrossRef CAS PubMed.
- J. Liu, J. Ni, Y. Zhao, H. Wang and L. Gao, J. Mater. Chem. A, 2013, 1, 12879 CAS.
- Z. X. Yang, G. D. Du, Q. Meng, Z. P. Guo, X. B. Yu, Z. X. Chen, T. L. Guo and R. Zeng, RSC Adv., 2011, 1, 1834–1840 RSC.
- Y. Luo, J. Luo, J. Jiang, W. Zhou, H. Yang, X. Qi, H. Zhang, H. J. Fan, D. Y. W. Yu, C. M. Li and T. Yu, Energy Environ. Sci., 2012, 5, 6559 CAS.
- D. Su, H.-J. Ahn and G. Wang, J. Mater. Chem. A, 2013, 1, 4845 CAS.
- T. X. T. Sayle, R. R. Maphanga, P. E. Ngoepe and D. C. Sayle, J. Am. Chem. Soc., 2009, 131, 6161 CrossRef CAS PubMed.
- A. L. M. Reddy, M. M. Shaijumon, S. R. Gowda and P. M. Ajayan, Nano Lett., 2009, 9, 1002 CrossRef CAS PubMed.
- H. Xia, M. Lai and L. Lu, J. Mater. Chem., 2010, 20, 6896 RSC.
- J. Y. Liao, D. Higgins, G. Lui, V. Chabot, X. Xiao and Z. Chen, Nano Lett., 2013, 13, 5467 CrossRef CAS PubMed.
- D. Larcher, D. Bonnin, R. Cortes, L. Rivals, L. Personnaz and J.-M. Tarascon, J. Electrochem. Soc., 2003, 150, A1643 CrossRef CAS PubMed.
- P. Wang, M. Gao, H. Pan, J. Zhang, C. Liang, J. Wang, P. Zhou and Y. Liu, J. Power Sources, 2013, 239, 466 CrossRef CAS PubMed.
- Y. NuLi, P. Zhang, Z. Guo, P. Munroe and H. Liu, Electrochim. Acta, 2008, 53, 4213 CrossRef CAS PubMed.
- M. S. Wu and P. C. J. Chiang, Electrochem. Commun., 2006, 8, 383 CrossRef CAS PubMed.
- H. Xiao, Y. Xia, W. Zhang, H. Huang, Y. Gan and X. Tao, J. Mater. Chem. A, 2013, 1, 2307 CAS.
- A. Brandt and A. Balducci, J. Power Sources, 2013, 230, 44 CrossRef CAS PubMed.
- X. Li, X. Huang, D. Liu, X. Wang, S. Song, L. Zhou and H. Zhang, J. Phys. Chem. C, 2011, 115, 21567 CAS.
- S. Laruelle, S. Grugeon, P. Poizot, M. Dollé, L. Dupont and J.-M. Tarascon, J. Electrochem. Soc., 2002, 149, A627 CrossRef CAS PubMed.
- Y. Wang, F. Su, J. Y. Lee and X. S. Zhao, Chem. Mater., 2006, 18, 1347 CrossRef CAS.
- T. M. Bond, J. C. Burus, D. A. Stevens, H. M. Dahn and J. R. Dahn, J. Electrochem. Soc., 2013, 160, A521 CrossRef CAS PubMed.
- A. J. Smith, J. C. Burus and J. R. Dahn, Electrochem. Solid-State Lett., 2010, 13, A177 CrossRef CAS PubMed.
- S.-W. Kim, D.-H. Seo, H. Gwon, J. Kim and K. Kang, Adv. Mater., 2010, 22, 5260 CrossRef CAS PubMed.
- Y. Hou, Y. Cheng, T. Hobson and J. Liu, Nano Lett., 2010, 10, 2727 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |