Organic–inorganic hybrid polyvinylidene fluoride–Co0.6Zn0.4Mn0.3Fe1.7O4 nanocomposite film with significant optical and magnetodielectric properties
Arti Gupta*a and
Ram Pal Tandonb
aDepartment of Physics & Astrophysics, University of Delhi, Delhi 110007, India. E-mail: artigupta80@gmail.com; Fax: +91-27-667061; Tel: +91-2766-7725 ext. 1367
bDepartment of Physics & Astrophysics, University of Delhi, Delhi 110007, India
First published on 6th January 2015
Abstract
In this paper, we illustrate the significant optical and magnetodielectric properties of the polyvinylidene difluoride–Co0.6Zn0.4Mn0.3Fe1.7O4 nanocomposite film, containing the optimum weight content (0.5%) of CZFMO nanoparticles (average particle size ∼ 20 nm). The photoluminescence spectra of the nanocomposite film clearly demonstrated stable and sharp luminescence peaks at 430 nm (indigo region), 458 nm (blue region) and 490 nm (green region), accompanied by considerably higher intensity, compared to the broad emission peak (450–550 nm) observed for pristine polyvinylidene difluoride film. The results of fluorescence spectroscopy confirmed those obtained in photoluminescence. Polymer nanoparticle surface interaction could be the possible mechanism behind the enhanced optical properties. Additionally, this nanocomposite film exhibited frequency independent, linear and negative magnetodielectric effect ∼0.08% at the maximum applied field of 5 Tesla.
1. Introduction
Organic–inorganic hybrid polymer–inorganic nanocomposite materials are extensively investigated in the literature1–3 owing to their potential applications in optoelectronics, magnetooptical storage, solar cells, light emitting diodes (LEDs), microwave absorbers, etc. Inorganic nanosized oxides such as ZnO, TiO2, Fe3O4, and organic polymers such as polyvinylidene difluoride (PVDF), polyvinyl alcohol (PVA), and polyethylene glycol (PEG)1,4–6 are often used in fabricating nanocomposite materials. Among the inorganic oxides, ZnO has been a potential choice due to its remarkable semiconducting, magnetic and optical properties,7–9 useful in various device applications. The photoluminescence (PL) spectra of ZnO exhibit a peak at ∼500 nm (yellow region), which even with significant reduction in crystallite size,7–9 does not shift down to blue region. Later, Abdullah et al.10 successfully demonstrated a stable luminescence peak at ∼465 nm (blue region) for ZnO–PEG nanocomposite, obtained by in situ growth of ZnO nanoparticles in PEG matrix, using the unbalanced precursor molarity approach. Similarly, enhancement in the optical properties of polymers was illustrated with the properly designed polymer nanoparticle surface interaction.11–13 For instance, Chao et al.13 showed the enhanced PL and electroluminescence (EL) properties of benzoxyl dendron-substituted polyfluorene, obtained by incorporating a small fraction of surface modified CdS nanoparticles in the polymer matrix. Notably, polyfluorene layers are the promising14–16 source of blue light emission in LEDs due to high PL efficiency. However, the organic chromophores in polymers tend to quench, leading to broad emission bands with low PL efficiency; this hampers their device applications. As indicated by these investigations, appropriate selection of inorganic nanofiller, its content, polymer matrix and polymer nanoparticle surface interaction extensively influence the optical properties of polymer–inorganic nanocomposite.Notably, in literature inorganic nanofillers leading to the enhanced optical properties1,3 of organic polymer matrix, are limited to the semiconductor quantum dots of PbSe, CdS, CdSe, etc. Moreover, these semiconductor quantum dots are always used in core–shell structure along with surface modification/engineering to protect their PL features. The major concern with these semiconductor based polymer nanocomposites is the toxicity of Pb, Se and Cd (doped ZnS1 is the only known example of nontoxic semiconductor, used in fabrication of polymer–inorganic nanocomposites), which restrains their commercial use. On the other hand, investigations1,3 on iron oxide based (Fe2O3, Fe3O4, (Ni, Zn)Fe2O4) polymer nanocomposites are typically focused on their applications in electrical/magnetic shielding, nonlinear optics, magnetic electrocatalysis, microwave absorption etc.,due to unique combination of electrical and magnetic properties in these materials.
Inorganic nanosized ferrites exhibit interesting optical properties, compared to their bulk counterparts and, addition of even a very small amount of inorganic ferrite nanoparticles to host organic polymer matrix can substantially enhance the optical properties of resulting nanocomposite. However, this is still away from practical realization and, thus, merits investigations.
In our earlier publication,17 we reported novel as well as interesting magnetic/magnetostrictive properties of bulk Co0.6Zn0.4Mn0.3Fe1.7O4 (CZFMO). Here, we report the enhanced optical properties of PVDF–CZFMO nanocomposite film, containing the optimum weight content (0.5%) of CZFMO nanoparticles. This nanocomposite film displayed stable and sharp luminescence peaks at 430 nm (indigo region), 458 nm (blue region) and 490 nm (green region) accompanied by higher intensity, compared to broad emission peak (450–550 nm) observed for pristine PVDF film. Furthermore, magnetodielectric (MD) studies on nanocomposite film illustrated frequency independent, linear and negative MD effect (∼0.08% at H = 5 T). Clearly, PVDF–CZFMO nanocomposite film has the crucial advantages of non-toxicity and simplicity of preparation procedure (discussed in the Experimental section).
Notably, the state of dispersion and the nature of the interface between nanoparticle and host matrix largely affect the polymer nanoparticle interaction and overall performance of polymer nanocomposite. Consequently, we found that higher particle loading (>0.5 wt%) of CZFMO nanoparticles in PVDF–CZFMO nanocomposite film, with agglomeration and inhomogeneous dispersion, results in broad emission peak with low intensity, almost identical to that of pristine PVDF film. Here onwards, the results obtained on optimum PVDF–CZFMO nanocomposite film are discussed unless otherwise specified.
2. Experimental
Preparation of CZFMO nanoparticles
CZFMO nanopowder was prepared using the chemical co-precipitation method. The raw nitrates Co(NO3)2, Mn(NO3)2, Zn(NO3)2 and FeCl3 were weighed in a stoichiometric ratio. The metal nitrate/chloride solutions were mixed using continuous stirring at 60 °C for 1 hour. Thereafter, sodium hydroxide (NaOH) 8 M solution with pH = 12–13 was added drop by drop with continuous stirring at 60 °C for 3 hours. After complete chemical reactions, a black precipitate was obtained which was washed several times with deionized (DI) water and dried in oven at 100 °C. From the TEM micrograph (Fig. 1(a)), CZFMO nanoparticles were found to be almost spherical in shape with narrow particle size distribution (average particle size ∼18 nm). Cubic spinel structure was identified from the XRD pattern (Fig. 1(b)) with five prominent peaks of high intensity values. The average particle size ∼20 nm was calculated from the XRD pattern using the Debye Sherrer formula for the most intense peak (311). Where, D is the crystallite size, λ is the wavelength of the X-ray source ∼0.154 nm, β is the full width at half maximum of the most intense peak (311) and θ is the Bragg's angle.
for the most intense peak (311). Where, D is the crystallite size, λ is the wavelength of the X-ray source ∼0.154 nm, β is the full width at half maximum of the most intense peak (311) and θ is the Bragg's angle.
Preparation of nanocomposite film
PVDF powder purchased from Alfa Aesar (United Kingdom) was used in sample preparation. 0.5 g of PVDF was dissolved in 15 mL (N,N) dimethylformamide (DMF from Alfa Aesar) with constant stirring to obtain a clear transparent solution. Later, weighed amount of CZFMO nanopowder (0.5 wt%) was dispersed in PVDF–DMF solution using ultrasonication for 3–4 hours at 60 °C, followed by stirring for 1–2 hours. PVDF–CZFMO solution was spin coated (angular velocity and time of 3000 rpm and 30 seconds, respectively) on thoroughly cleaned glass substrate. Nanocomposite film was thermally annealed at 150 °C for 2 h and, then 250 °C for 30 minutes, with slow cooling down to room temperature. The pristine PVDF film was prepared under the same conditions. Thickness of pristine PVDF as well as nanocomposite films was found to be ∼400 nm. For dielectric, magnetic and MD characterizations, thin sheets (thickness ∼100 μm) of nanocomposite and pristine PVDF, were chosen. In order to obtain thin sheets, PVDF and PVDF–CZFMO solutions were individually poured on thoroughly cleaned glass slides. After complete evaporation of solvent, freely standing thin sheets were peeled off from glass slides and annealed under the aforementioned conditions.Characterization
XRD patterns were taken using Rigaku X-ray diffractometer with CuKα radiation of wavelength = 0.154 nm. The high resolution transmission electron micrograph (HRTEM) was taken using TECNAI G2 T30, U-TWIN transmission electron microscope operated at 50–300 keV. The low temperature magnetic measurements in ZFC and FC conditions were performed using Physical Property Measurement System (PPMS) set up available at AIRF-JNU, New Delhi (India). For ZFC data sample is cooled in the zero field and magnetization is measured during heating the sample in the presence of the applied field, whereas for the FC data sample is cooled in the presence of an applied field and magnetization is measured during heating the sample in the presence of field. FTIR spectra were taken using the SPECTRUM XIFT IR (Perkin Elmer) (650–4000 cm−1) in the Attenuated Total Reflectance (ATR) mode. Raman spectra were taken using Renishaw inVia (100–3200 cm−1) with excitation wave length ∼532 nm. Optical absorption studies were made using (Hitachi U-3900 H/Ocean Optics HR-4000) and PL measurements were done using Horiba jobin yvon Fluorolog-3. Varian CARY Eclipse Fluorescence Spectrophotometer was employed to perform fluorescence measurements. The surface topographical images were taken using Nanosurf Easyscan 2 Controller, Atomic Force Microscope. Surface scanning electron micrographs (SEM) were taken using JEOL 6610LV scanning electron microscope. Dielectric constant spectra were taken using an impedance analyzer (HP 4294 A, Agilent, CA) at room temperature in the frequency range (100 Hz–110 MHz). Polarization (P) vs. electric field (E) loop measurements were done using Precision Premier II ferroelectric loop tester from Radiant Technology. For dielectric, P–E and MD measurements, electrical contacts were made on both sides of the sheet using conducting silver paste (dried at room temperature).3. Results and discussion
FTIR spectra (Fig. 2) for PVDF (solution form) showed absorption bands at 660, 742, 811, 830, 882, 1063, 1084, 1100, 1200, 1234, 1385 and 1403 cm−1.18–20 Bands at 811, 830, 882 and 1234 cm−1 can be ascribed to the γ phase of PVDF, whereas, remaining bands can be assigned to the α phase of PVDF. Bands of β and γ phases of PVDF usually appear close to each other and sometimes, cannot be distinguished from each other. For instance, band at 882 cm−1 could be indicative of either β or γ phase of PVDF. However, band at 1234 cm−1 can be clearly assigned to the γ phase of PVDF. High energy supplied during the sonication process could change the polymer chain conformation and induce γ phase under these conditions. Thus, a mixture of α and γ phases was found in PVDF solution. For nanocomposite (solution form), FTIR spectra showed bands at 660, 742, 811, 830, 882, 1060, 1086, 1100, 1200, 1234, 1379 and 1402 cm−1, indicating the mixture of α and γ phases. For PVDF, absorption band at 1385 cm−1 with a shoulder at 1403 cm−1 can be assigned to the CF2 stretching.21 For nanocomposite, two separate bands at 1376 cm−1 (shifted compared to that of PVDF) and 1403 cm−1 with almost equal intensities were noticed. The change in position as well as the intensity of bands related to C–F bond implies the significant interaction between the surface charge on CZFMO nanoparticles (with high surface area) and CF2 dipoles of PVDF chains. For PVDF–CZFMO nanocomposite, the polymer nanoparticle surface interaction substantially changes the polymer chain conformation close to the surface of nanoparticle and induces γ α phase transformation. This is anticipated due to relatively higher content of α phase (estimated from this FTIR data) in nanocomposite. Interestingly, contrary to other nanocomposites22–24 (showing higher content of γ phase), PVDF–CZFMO nanocomposite exhibited higher content of α phase. The possible cause of high α phase content is discussed later.Surface topographical images of nanocomposite and pristine PVDF films are shown in Fig. 3(a) and (b), respectively. Topography of nanocomposite film is significantly different from pristine PVDF film due to embedded CZFMO nanoparticles. Root mean square roughness (RMS) or Sq. are 137.64 nm and 288.11 nm for pristine PVDF and nanocomposite films, respectively (in the spin coating method high humidity and low substrate temperature lead25 to highly rough PVDF films). Higher surface roughness for nanocomposite film could be an outcome of stress/phase transformation induced by particle loading. Surface SEM images for pristine PVDF and nanocomposite films are shown in Fig. 4(a) and (b), respectively. For pristine PVDF film, tree like spherulites were observed, which grew from their primary nucleation sites, developed during the spin coating process. For nanocomposite film, CZFMO nanoparticles can be identified as bright dots embedded in the dark (appearing dark due to high resistivity) PVDF matrix. This further confirms the formation of nanocomposite film without any detectable porosity. Comparison of surface SEM images for pristine PVDF and nanocomposite films indicate higher roughness for nanocomposite film, consistent with AFM data. Particle size of CZFMO estimated from this SEM image is roughly in agreement with that determined using the TEM micrograph.
Raman spectra for pristine PVDF and nanocomposite films are shown in Fig. 5. Broad Raman bands with low intensity are characteristics of semicrystalline PVDF. For both samples, three Raman bands at 564 cm−1 (ν1), 801 cm−1 (ν2) and 1094 cm−1 (ν3), were observed. Band (ν1) at 564 cm−1 appeared at an intermediate position between the band at 538 cm−1, related to the CF2 deformation mode and band at 609 cm−1, related to the CF2 wagging mode.26,27 In literature,28,29 band at 794 cm−1 is attributed to α phase of PVDF, whereas, band at 811 cm−1 is attributed to γ phase of PVDF. For our samples, the appearance of the Raman band at an intermediate position of 801 cm−1 possibly indicates the presence of mixture of α and γ phases. Band at 1094 cm−1 (ν3)28,29 can originate due to a combination of different phases of PVDF. Two noticeable changes found in the spectra for nanocomposite film, were as following: (i) intensity ratio (Iν3/Iν1) increases (ii) peak (ν2) becomes sharp and intense (without any detectable change in the position). These changes signify the effectiveness of the polymer nanoparticle surface interaction.
The optical absorption spectra in the wavelength range (200–600 nm) for pristine PVDF and nanocomposite films are shown in Fig. 6(a). The absorption edge was found at ∼322 nm for pristine PVDF and at ∼335 nm for nanocomposite film. Inclusion of CZFMO nanoparticles in PVDF matrix increases absorption and shifts the position of absorption edge towards higher wavelength or lower frequency side, demonstrating red shift in spectra. Transmittance (%) vs. wavelength (nm) plots for PVDF and nanocomposite films are shown in Fig. 6(b). Significant reduction in transmittance for nanocomposite film is evident. For polymer nanocomposites, transmittance is largely affected by the surface roughness, particle size, dispersion of nanoparticles, polymer nanoparticle interface, refractive index, etc. For nanocomposite film, decrease in transmittance can be caused4,30 by Rayleigh scattering (by CZFMO nanoparticles) and relatively high surface roughness (evident from the AFM and surface SEM images). The optical band gap energy was estimated using the following Tauc relation31
| (αhν)1/n = A(hν − EG) | (1) |
 | ||
| Fig. 6 UV-visible spectra for pristine PVDF and nanocomposite films (a) absorbance and (b) transmittance. | ||
The PL spectra (excitation wavelength = 400 nm) for pristine PVDF and nanocomposite films are shown in Fig. 8. Nanocomposite film displayed stable and sharp luminescence peaks at 430 nm (indigo region), 458 nm (blue region) and 490 nm (green region), accompanied by higher intensity, compared to broad32 emission peak (450–550 nm) observed for pristine PVDF film. The first band at 430 nm (∼3 eV) is close to the direct band gap energy value (estimated from optical absorption spectra) and could be attributed to the band edge emission. The second band at 458 nm (2.7 eV) could be ascribed to the surface trap states associated with nanoparticles, whereas, the third band at 490 nm (2.5 eV) could be attributed to the deep trap states. The PL spectra for CZFMO nanopowder (inset of Fig. 8) demonstrated a broad emission peak (420–440 nm). This broad emission peak arises due to possible overlap of d–d transitions 4E, A1(4G) 6A1(6S). For nanocomposite film, PL features like sharp emission peaks with high intensity are unlike those observed for both PVDF and CZFMO. Enhanced PL efficiency for nanocomposite film should be caused by the polymer nanoparticle surface interaction. This interaction involves the electric field produced by the surrounding highly electronegative CF2 dipoles of polymer chains, that could promote the 3d–4s 4p orbital (4s and 4p orbitals are hybridized) coupling. Such coupling gives rise to populated 4s and 4p orbitals and delocalization of electrons.33 Consequently, electrons from 4s and 4p orbitals take part in the band edge transitions. This resulted in stronger PL intensity for nanocomposite film.
 | ||
| Fig. 8 PL spectra for pristine PVDF and nanocomposite films. inset shows PL spectra for CZFMO nanopowder. | ||
The fluorescence spectra for PVDF and nanocomposite (solution form) are shown in Fig. 9(a). For nanocomposite, the spectra illustrated three emission bands at 430 nm, 458 nm and 490 nm (appearing as a shoulder), accompanied by significantly higher intensity, compared to broad emission peak (450–550 nm) observed for PVDF. Clearly, the results of fluorescence spectroscopy are in complete agreement with those obtained in PL. In order to probe the dynamic behavior of involved electronic transitions, time resolved fluorescence spectroscopy (TRFS) (Fig. 9(b)) was employed. Notably, the mean lifetime of exciton, determined using TRFS, gives useful information regarding the quality of material and its device performance. The decay profile was fitted using three exponentials for PVDF and using two exponentials for nanocomposite. Using this data, the mean life time was determined to be ∼1.61 × 10−9 s (χ2 = 1.03) and 1 × 10−10 s (χ2 = 0.96) for PVDF and nanocomposite, respectively. Since, the efficiency of radiative recombination is inversely proportional to the decay time, more (roughly by an order of magnitude) efficient recombination could be anticipated for nanocomposite (attractive feature for device applications).
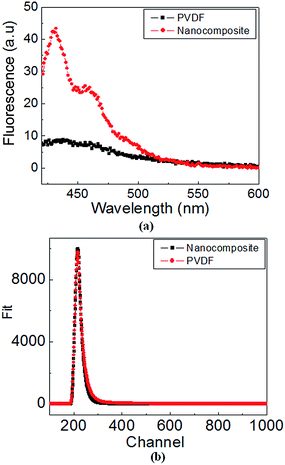 | ||
| Fig. 9 (a) fluorescence spectra for pristine PVDF and nanocomposite (solution form) (b) time resolved fluorescence data. | ||
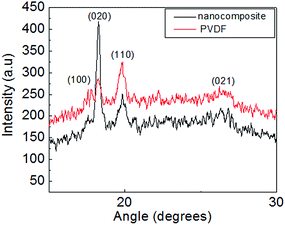
The XRD patterns for pristine PVDF and nanocomposite (sheet form) are shown in Fig. 10. For pristine PVDF, four peaks at ∼17.6°, 18.2°, 19.8° (most intense) and 26.7° corresponding to (100), (020), (110) and (021) crystalline planes of α phase of PVDF,29 were noted. Whereas, the XRD pattern for nanocomposite showed a striking difference; peak at 18.2° corresponding to (020) plane has the highest intensity, unlike that observed for pristine PVDF (peak positions are essentially unchanged). Interestingly, Steinhart et al.34 first reported such observation for nanotubes consisting of α-PVDF; therein, XRD pattern revealed the b-axis oriented crystallites of PVDF inside the wall of the nanotube (b-axis of unit cell was oriented parallel to the long axis of the nano tube). For PVDF–CZFMO nanocomposite, the substantial change in XRD pattern signifies the strength of polymer nanofiller surface interaction. We believe that the pores on the surface of spherical CZFMO nanoparticle can act as templates and promote the oriented growth of α-PVDFcrystallites (depending on the geometry of the pores, i.e. shape, size etc.) in nanocomposite. Evidently, the growth took place along the b-axis (major growth axis), giving a manifestation of anisotropy in nanocomposite, unlike the isotropic reflections observed for pristine PVDF.
Magnetization (M) vs. temperature (T) plots for nanocomposite in ZFC and FC conditions in the temperature range (2 K ≤ T ≤ 300 K) at H = 1 T are shown in Fig. 11. At high magnetic field of 1 T, complete alignment of individual magnetic moments of CZFMO nanoparticles in the direction of applied field takes place. Consequently, in the entire temperature range of investigation, ZFC and FC plots completely overlapped each other, demonstrating the existence of long range magnetic ordering. Magnetization decreases with the increase in temperature due to the added thermal energy, which breaks the collinear arrangement of magnetic spins. Clearly, the M–T plot showed the normal expected ferrimagnetic35 behavior, confirming the magnetic ordering of dispersed CZFMO nanoparticles (the superparamagnetic behavior of CZFMO nanoparticles is reported in our recent publication36).
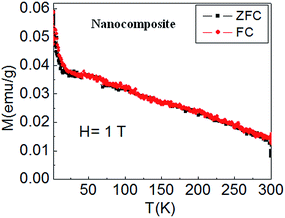 | ||
| Fig. 11 Magnetization (M) vs. temperature (T) plots for nanocomposite sheet in ZFC and FC conditions at H = 1 T. | ||
The dielectric constant (ε′) vs. frequency (f) plots at room temperature in the frequency range (100 Hz–110 MHz) for pristine PVDF and nanocomposite are shown in Fig. 12. In the entire frequency range, ε′ and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ for nanocomposite are higher than those of pristine PVDF. Similar observations were reported in literature for other PVDF based nanocomposite systems.37,38 For present instance, enhancement in ε′ could be attributed to the presence of oriented crystallites of PVDF in nanocomposite. Similarly, higher tan
δ for nanocomposite are higher than those of pristine PVDF. Similar observations were reported in literature for other PVDF based nanocomposite systems.37,38 For present instance, enhancement in ε′ could be attributed to the presence of oriented crystallites of PVDF in nanocomposite. Similarly, higher tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ for nanocomposite reflects the difference between oriented and unoriented form of α-PVDF crystallites. Here, the ε′–f plots also revealed the thickness resonance mode for pristine PVDF and nanocomposite. The resonance (fR) and antiresonance (fA) frequencies for pristine PVDF were found to be 83 MHz and 99 MHz, respectively, with bandwidth (Δf = fA − fR) value of 13 MHz. For nanocomposite, a considerable increase in value of tan
δ for nanocomposite reflects the difference between oriented and unoriented form of α-PVDF crystallites. Here, the ε′–f plots also revealed the thickness resonance mode for pristine PVDF and nanocomposite. The resonance (fR) and antiresonance (fA) frequencies for pristine PVDF were found to be 83 MHz and 99 MHz, respectively, with bandwidth (Δf = fA − fR) value of 13 MHz. For nanocomposite, a considerable increase in value of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ close to resonance, was noted, however, the values of fA, fR and Δf are essentially same as pristine PVDF.
δ close to resonance, was noted, however, the values of fA, fR and Δf are essentially same as pristine PVDF.
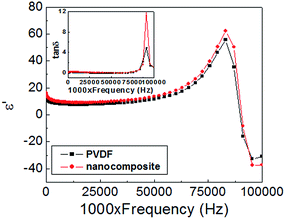 | ||
Fig. 12 Dielectric constant spectra for pristine PVDF and nanocomposite sheets in the frequency range (100 Hz–110 MHz) at room temperature. Inset shows corresponding tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ vs. frequency plots. δ vs. frequency plots. | ||
P–E loops for pristine PVDF and nanocomposite are shown in Fig. 13. Evidently, loops do not saturate up to the maximum applied electric field of 200 kV cm−1. For pristine PVDF, the maximum polarization (Pmax), remnant polarization (PR) and coercivity (EC) were observed to be 0.116 μC cm−2, 0.015 μC cm−2 and 24.9 kV cm−1, respectively. Whereas, for nanocomposite, Pmax, PR and EC were observed to 0.134 μC cm−2, 0.016 μC cm−2, 23 kV cm−1, respectively. Higher polarization values (Pmax & PR) for nanocomposite can be ascribed to the presence of oriented crystallites of α-PVDF (concurrently with the dielectric data).
 | ||
| Fig. 13 Polarization (P) vs. electric field (E) loops at room temperature for pristine PVDF and nanocomposite sheets. | ||
MD plots for nanocomposite at two different frequencies 30 kHz and 300 kHz are shown in Fig. 14. MD effect basically probes the coupling between magnetization and polarization in a magnetoelectric material. Change in dielectric constant (ε′) on application of magnetic field, is measured in term of MD effect, defined as [ε′(H) − ε′(0)/ε′(0)%]. Where ε′(H) is the permittivity in the presence of field H and ε′(0) is the permittivity at H = 0 Oe. The frequency independent MD effect observed for nanocomposite confirmed the absence of extrinsic contribution from magnetoresistance together with Maxwell–Wagner polarization. MD (%) almost linearly decreases with the increase in field, attaining the maximum MD ∼0.08% at the highest applied field of 5 T. In literature, negative MD effect was demonstrated for PVDF–Ni0.5Zn0.5Fe2O4 (PVDF–NZFO) nanocomposite films.39 Whereas, the positive MD effect was shown40 for PVDF–Fe3O4 nanocomposite film.40 Considering the magnitudes, maximum MD = 5% (at the maximum applied field ∼1 T) was obtained in PVDF–NZFO nanocomposite film, containing 20 wt% loading of NZFO nanoparticles and, maximum MD = 0.6% (at the maximum applied field ∼2 T) was obtained in PVDF–Fe3O4 nanocomposite film, containing 9.09 wt% of Fe3O4 nanoparticles. Low value of MD (∼0.08% at the highest applied field of 5 T) for PVDF–CZFMO nanocomposite can be ascribed to the small loading (0.5 wt%) of CZFMO nanoparticles.
 | ||
| Fig. 14 Room temperature MD% vs. field (H) plots in the field range (−5 T ≤ H ≤ 5 T) for nanocomposite sheet at two different frequencies of 30 kHz and 300 kHz. | ||
Importantly, PVDF–CZFMO nanocomposite displayed linear MD effect unlike the nonlinear MD effect observed for other nanocomposites.39,40 The mechanism behind the linear MD effect can be explained as following: when magnetic field is applied, the spins of CZFMO nanoparticles orient themselves along the direction of the field. Subsequently, the spin–charge coupling between polymer and nanoparticle redistributes the charge on the dipoles of polymer chains, leading to a change in polarization/capacitance. This spin–charge coupling between CZFMO nanoparticles and PVDF chains induces linear change in dielectric constant with the change in magnetization. Thus, it is proportional to the P2M term in the expression of free energy, unlike the dominant contribution of quadratic P2M2 term (arising from the strain mediated mechanical coupling between piezoelectric and magnetostrictive phases) considered for PVDF–Fe3O4 and PVDF–NZFO nanocomposites.39,40
4. Conclusion
In this work, we report the enhanced optical properties of PVDF–CZFMO nanocomposite, containing the optimum weight content (0.5 wt%) of CZFMO nanoparticles. Polymer nanoparticle surface interaction can be the possible mechanism behind the enhanced optical properties. Additionally, this nanocomposite exhibited frequency independent, linear and negative MD effect (0.08% at H = 5 T). Thus, PVDF–CZFMO nanocomposite could be seen as a new smart material for various device applications.One of the authors (A.G) would like to thank DST-INSA for providing Inspire Faculty Fellowship. We would like to thank Prof. S. Patnaik at JNU (India) for MD measurements.
References
- S. Li, M. M. Lin, M. S. Toprak, D. K. Kim and M. Muhammed, Nano Rev., 2010, 1, 5214 CrossRef PubMed.
- X. M. Sui, C. L. Shao and Y. C. Liu, Appl. Phys. Lett., 2005, 87, 113115 CrossRef PubMed.
- J. Z. Zhang, Optical Properties and spectroscopy of Nanomaterials, World Scientific Pub, 2009 Search PubMed.
- A. P. Indolia and M. S. Gaur, J. Polym. Res., 2013, 20, 43 CrossRef.
- A. L. Santiago, H. R. Grant, P. Gangopadhyay, R. Voorakaranam, R. A. Norwood and N. Peyghambarian, Opt. Mater. Express, 2012, 2, 978 CrossRef.
- L. Beecroft and C. M. Ober, Chem. Mater., 1997, 9, 1302 CrossRef CAS.
- C. Xu, C. Ouyang, R. Jia, Y. Li and X. Wang, J. Appl. Polym. Sci., 2009, 111, 1763 CrossRef CAS.
- L. Spanhel and M. A. Anderson, J. Phys. Chem. B, 1991, 113, 2826 CAS.
- S. A. Studenikin, N. Golego and M. Cocivera, J. Appl. Phys., 1998, 84, 2287 CrossRef CAS PubMed.
- M. Abdullah, T. Morimoto and K. Okuyama, Adv. Funct. Mater., 2003, 13, 800 CrossRef CAS.
- H. Yang and P. H. Holloway, J. Phys. Chem. B, 2003, 107, 9705 CrossRef CAS.
- Y. H. Kim, D. Kim, S. C. Jeoung, J. Y. Han, M. S. Jang and H. K. Shim, Chem. Mater., 2001, 14, 643 Search PubMed.
- C. H. Chao, H. S. Wang, K. H. Wai and J. Y. Huang, Adv. Funct. Mater., 2006, 16, 909 CrossRef.
- I. Prieto, J. Teetsov, M. A. Fox, D. A. V. Bout and A. J. Bard, J. Phys. Chem. A, 2001, 105, 520 CrossRef CAS.
- Q. Pei and Y. Yang, J. Am. Chem. Soc., 1996, 118, 7416 CrossRef CAS.
- D. Y. Kim, H. N. Chao and C. Y. Kim, Prog. Polym. Sci., 2000, 25, 1089 CrossRef CAS.
- A. Gupta, A. Huang, S. R. Sannigrahi and R. Chatterjee, Appl. Phys. Lett., 2011, 98, 112901 CrossRef PubMed.
- A. Salimi and A. A. Yousefi, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 3487 CrossRef CAS.
- Y. J. Park, Y. S. Kang and C. Park, Eur. Polym. J., 2005, 41, 1002 CrossRef CAS PubMed.
- L. Mathias, R. Davis and W. Jarrett, Macromolecules, 1999, 32, 7958 CrossRef CAS.
- M. Baican, E. Paslarua, E. G. Hitruca and C. Vasilea, Dig. J. Nanomat. and Biostruc., 2011, 6, 1053 Search PubMed.
- T. Fornes and D. Paul, Polymer, 2003, 44, 3945 CrossRef CAS.
- D. Lincoln, R. Vaia and R. Krishnamoorti, Macromolecules, 2004, 37, 4554 CrossRef CAS.
- C. L. Liang, Z. H. Mai, Q. Xie, R. Y. Bao, W. Yang, B. H. Xie and M. B. Yang, J. Phys. Chem. B, 2014, 118, 9104 CrossRef CAS PubMed.
- M. Li, I. Katsouras, C. Piliego, G. Glasser and I. Lieberwirth, J. Mater. Chem. C, 2013, 1, 7695 RSC.
- M. J. Hannon, B. J. Boerio and J. L. Koenig, J. Chem. Phys., 1969, 50, 2829 CrossRef CAS PubMed.
- J. L. Koenig, Spectroscopy of Polymers, Elsevier, New York, 1999 Search PubMed.
- Y. Bormashenko, R. Pogreb, O. Stanevsky and E. Bormashenko, Polym. Test., 2004, 23, 791 CrossRef CAS PubMed.
- S. Satapathy, S. Pawar, P. K. Gupta and K. B. R. Verma, Bull. Mater. Sci., 2011, 34, 727 CrossRef CAS PubMed.
- P. I. Devi and K. Ramchandran, J. Exp. Nanosci., 2011, 6, 281 CrossRef CAS.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi, 1966, 15, 627 CrossRef CAS.
- E. Peik, J. Phys. D: Appl. Phys., 2007, 40, 3330 CrossRef CAS.
- H. Fei, X. Ai, M. Gao, Y. Yang, T. Zhang and J. Shen, J. Lumin., 1996, 66–67, 345 Search PubMed.
- M. Steinhart, S. Senz, R. B. Wehrspohn, U. Gosele and J. H. Wendorff, Macromolecules, 2003, 36, 3646 CrossRef CAS.
- A. Goldman, Modern Ferrite Technology, Van Nostrand Reinhold, New York, 1990, vol. 35 Search PubMed.
- A. Gupta and R. P. Tandon, Mater. Res. Soc. Symp. Proc., 2014, 1708 DOI:10.1557/opl.2014.607.
- P. Martinsa, C. M. Costaa, G. Botelhob, S. Lanceros-Mendeza, J. M. Barandiaranc and J. Gutierrezc, Mater. Chem. Phys., 2012, 131, 698 CrossRef PubMed.
- R. Gonçalves, P. M. Martins, C. Caparrós, P. Martins, M. Benelmekki, G. Botelho, S. Lanceros-Mendez, A. Lasheras, J. Gutiérrez and J. M. Barandiarán, J. Non-Cryst. Solids, 2013, 361, 93 CrossRef PubMed.
- Y. Guo, Y. Liu, J. Wang, R. L. Withers, H. Chen, L. Jin and P. Smith, J. Phys. Chem. C, 2010, 114, 13861 CAS.
- O. P. Jaykumar, B. P. Mandal, J. Majeed, G. Lawes, R. Naik and A. K. Tyagi, J. Mater. Chem. C, 2013, 1, 3710 RSC.
| This journal is © The Royal Society of Chemistry 2015 |