Novel and processable polyimides with a N-benzonitrile side chain: thermal, mechanical and gas separation properties
Mohammad Dinari* and
Hashem Ahmadizadegan
Department of Chemistry, Isfahan University of Technology, Isfahan, 84156-83111, Iran. E-mail: dinari@cc.iut.ac.ir; mdinary@gmail.com; Fax: +98 3133912351; Tel: +98 3133913270
First published on 4th March 2015
Abstract
A series of organo-soluble and thermally stable polyimides (PIs) with a N-benzonitrile side group were synthesized from a new diamine monomer, 3-(bis(4-aminophenyl)amino)benzonitrile with four different aromatic dianhydrides via a thermal or chemical imidization method. The chemical structure and purity of the synthesized compounds were determined by different techniques. The inherent viscosities of the resulting PIs were in the range of 0.97–1.13 dL g−1 at a concentration of 0.5 g dL−1 in DMAc at 30 °C and these polymers were easily dissolved in organic solvents such as N-methyl-2-pyrrolidone, N,N-dimethylacetamide, and N,N-dimethylformamide, and tetrahydrofuran at room temperature. The obtained PIs showed a reasonably high glass-transition temperature (Tg up to 291 °C) and high thermal stability (T10 up to 420 °C). Flexible and transparent films were obtained easily by solution casting. The membranes of these polymers showed a tensile strength up to 115 MPa with elongation at break up to 13%, low water absorption (0.32–0.88%), low dielectric constant (1.98–2.38 at 1 MHz) and high optical transparency (λcut-off up to 352 nm). The combination of excellent solubility, film quality, and thermal stability makes these PIs potential candidates for high-performance gas separation membrane applications using a solution-casting processes. Permeability measurements were made for O2, H2, CO2 and CH4 at 35 °C.
1. Introduction
Aromatic polyimides (PI)s are a class of thermally stable polymers due to their astonishing thermal and chemical resistance, good film forming ability, low dielectric constant, superior mechanical and physical properties as well as excellent electrical properties.1–4 PIs also possess outstanding dimensional stability under loads, which allows their use in high-temperature environments. Certainly, the presence of imide rings and subsequently charge transfer complexes in the polymer backbone lead directly to a favorable balance of chemical and mechanical properties.5–8 Because of the ability to maintain their properties in such a wide temperature range, PIs are applied in many industrial applications including the aerospace industry as constitutional materials and structural parts, insulation materials, hot zone resins, adhesives; in the electronic and microelectronic industry as dielectric materials, liquid crystal displays, memory devices, printed circuit boards, cable jackets, and wire coatings.9–13 The functional nitrile groups may lead to the increase of thermooxidative resistance and of the dielectric constant relative to the polymers that do not have this substituent on to the macromolecular chain. Also, the nitrile substituent may increase other physical properties of high performance materials, since it was shown that some amorphous polymers containing strong dipoles displayed a piezoelectric response.14–17 PIs are also commonly used as gas separation and fuel cell membranes, automotive components, medical tubing, humidity sensors, as well as fiber optic coatings.17PI based gas separation membranes are anticipated to contribute in numbers of major environmental and energy technologies including gas purification and capture.18 The requirement of high permeability and good selectivity is then considered as a main factor for the design and preparation of high performance gas separation membranes.19 There is an inherent trade-off of gas pairs between permeability and selectivity for entirely the gas separation polymer membranes, which was quantified in 1991 and 2008 by Robeson.20,21 The upper bound was identified on plots of double logarithmic plots of selectivity against permeability for a wide range of gas pairs using a comprehensive list of existing polymer permeability.20,21
To obtain high performance PIs materials by structural modification, a high-purity 3-[bis(4-aminophenyl)amino]benzonitrile, was synthesized through a modificatory method and subsequently polycondensed with various commercially available aromatic dianhydrides including as 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA or a), 4,4′-benzophenone tetracarboxylicdianhydride (BTDA or b), 4,4′-oxydiphthalic dianhydride (ODPA or c) and 3,3′,4,4′-diphenylsulfone tetracarboxylic dianhydride (DSDA or d) to produce a series of PIs containing benzonitrile units. It is hoped that these benzonitrile-containing PIs will have enhanced processability and enhanced thermal stability. The characterizations of the diamine and related intermediates, as well as the resulting polymers based on the diamine were carried out by means of elemental analyses, Fourier transform infrared (FT-IR), proton nuclear magnetic resonance (1HNMR) spectroscopy, X-ray diffraction (XRD), thermogravimetric analysis (TGA), mechanical testing and differential scanning calorimetry (DSC) techniques. Also the dielectric properties, optical transparency and gas separation properties of these novel materials were studied.
2. Experimental
2.1. Materials
All materials and solvents were purchased from Merck Chemical Co and Aldrich Chemical CO. 3-Aminobenzonitrile and 1-fluoro-4-nitrobenzene, palladium on activated carbon (10 wt%), hydrazine hydrates were used as received. All the dianhydrides were recrystallized from acetic anhydride and heated at 150 °C overnight prior to use. N-Methyl-2-pyrrolidone (NMP), N,N-dimethylformamide (DMF) and N,N′-dimethylacetamide (DMAc) were dried over barium oxide, followed by fractional distillation.2.2. Synthesis of diamine monomer
In a 50 mL three-neck round-bottom flask was placed 3-aminobenzonitrile (1 g, 8.46 mmol), 1-fluoro-4-nitrobenzene (2.41 g, 17.10 mmol), cesium fluoride (2.56 g, 19 mmol), and 20 mL of DMSO. The mixture was heated with stirring at 110 °C for 5 h under nitrogen. The reaction mixture was cooled and then poured in to 250 mL of ethanol. The yellow precipitate was collected by filtration and dried under vacuum. The product was purified by recrystallization from glacial acetic acid to afford 3-(bis(4-nitrophenyl)amino)benzonitrile in 90% yield; mp 218–220 °C. Hydrazine monohydrate (5 mL) was added dropwise to a mixture of dinitro compound (1.0 g, 2.77 mmol) and a catalytic amount of 10% palladium on activated carbon (Pd/C, 0.02 g) in ethanol (30 mL) at the boiling temperature. The mixture became homogeneous after 1.5 h, and the reaction was refluxed for 30 h. The mixture was then filtered to remove Pd/C. After cooling, the precipitated blue crystals were isolated by recrystallization from ethanol. The yield was 81%; mp 188–191 °C.FTIR (KBr, cm−1) of diamine: 3448 (s), 3377 (s), 3113 (w), 3075 (w), 2225 (s), 1555 (m), 1535 (m) 1440 (w), 1323 (w), 1252 (w), 844 (m), 739 (w).
1H-NMR (500 MHz, DMSO-d6, ppm) of diamine: 4.49 (s, 4H, NH), 6.26–6.28 (d, 8H, Ar-H, J = 4.5 Hz), 6.49 (s, 1H, Ar-H), 6.76–6.78 (d, 1H, Ar-H), 7.19–7.20 (d, 1H, Ar-H, J = 4 Hz), 7.36–7.38 (dd, 1H, Ar-H, J = 4 Hz).
13C-NMR (125 MHz, DMSO-d6), of diamine, δ (ppm): 108.22 (Ar), 112.31 (CN), 118.82 (Ar), 121.18 (Ar), 123.32 (Ar), 124.12 (Ar), 125.17 (Ar), 137.25 (Ar), 138.45 (Ar), 141.35 (Ar), 148.33 (Ar).
Elemental analysis calculated for C19H16N4 (300.36 g mol−1): calcd (%) C, 75.98%; N, 18.65%; H, 5.37%. Found (%) C, 75.86%; N, 18.79%; H, 5.39%.
2.3. Synthesis of high performance PIs
Novel PIs (a–d) were synthesized by the reactions of diamine with different dianhydrides (a–b). The synthesis of PIa is employed as an example to show the general synthetic method employed to prepare the PIs. A mixture consisting of 1.00 g (3.32 mmol) of diamine, 1.47 g (3.32 mmol) of 6FDA, and 100 mL of NMP were added to a 250 mL three-necked flask which was equipped with a mechanical stirrer, a condenser and a nitrogen inlet. The reaction mixture became very viscous after 2 h. The mixture was constantly stirred for 2 h at room temperature to give poly(amic acid) (PAA). In the next step, 16.8 mL of triethylamine in 50 mL of NMP was added slowly to the reaction mixture, which was stirred for another 4 h at room temperature. The inherent viscosity of the PAAa was 1.15 dL g−1, as measured in DMAc at a concentration of 0.5 g dL−1 at 30 °C. The PAA was converted into PI with either a thermal or chemical imidization method. In the thermal imidization method, about 2 g of the PAA solution was spread into a Petri culture dish 7 cm in diameter and baked at 90 °C overnight (ca. 12 h) for the removal of the casting solvent. The semidried PAA film was further dried and converted into the PI by sequential heating at 150 °C for 30 min, at 200 °C for 30 min, and at 250 °C for 1 h. For the elemental analysis, tensile testing, X-ray crystallography, and thermal analysis, the PI films were further heated at 350 °C for another 1 h to ensure the complete imidization. In the chemical imidization method, 5 mL of acetic anhydride and 2 mL of pyridine were added to the remaining PAA solution, and the mixture was heated at 100 °C for 1 h to affect a complete imidization. The resultant solution of the polymer was poured slowly into 250 mL of methanol, and the precipitate was washed thoroughly with methanol and hot water, collected by filtration, and dried in air at 150 °C for 4 h.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1775 (asymmetric imide C
N), 1775 (asymmetric imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1722 (symmetric imide C
O stretching), 1722 (symmetric imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1625 (aromatic C
O stretching), 1625 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching), 1236, 1126 (C–F stretching), and 821 (C–N bending).
C stretching), 1236, 1126 (C–F stretching), and 821 (C–N bending).1H-NMR (500 MHz, DMSO-d6, ppm): 6.26–6.27 (d, 2H, Ar-H, J = 3.5), 6.48–6.49 (s, 2H, Ar-H, J = 4.5 Hz), 6.68–7.69 (d, 2H, Ar-H, J = 4.5 Hz), 6.88 (s, 1H, Ar-H), 7.27–7.28 (d, 1H, Ar-H, J = 3.5), 7.36–7.37 (d, 1H, Ar-H, J = 3.5), 7.17–7.18 (d, 1H, Ar-H, J = 4.5 Hz), 7.66–7.68 (d, 1H, Ar-H, J = 3.5), 7.88–7.89 (d, 1H, Ar-H, J = 4.5 Hz), 8.17–8.18 (d, 1H, Ar-H, J = 4.5 Hz), 8.38–8.39 (d, 1H, Ar-H, J = 4.5 Hz).
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1777 (asymmetric imide C
N), 1777 (asymmetric imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1726 (symmetric imide C
O stretching), 1726 (symmetric imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1629 (aromatic C
O stretching), 1629 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching), 825 (C–N bending).
C stretching), 825 (C–N bending).1H-NMR (500 MHz, DMSO-d6, ppm): 6.15–6.16 (d, 2H, Ar-H, J = 3.5), 6.55–6.57 (s, 2H, Ar-H, J = 4.5 Hz), 6.62–7.64 (d, 2H, Ar-H, J = 4.5 Hz), 6.71 (s, 1H, Ar-H), 7.45–7.46 (d, 1H, Ar-H, J = 3.5), 7.53–7.55 (d, 1H, Ar-H, J = 3.5), 7.78–7.80 (d, 1H, Ar-H, J = 4.5 Hz), 7.82–7.83 (d, 1H, Ar-H, J = 3.5), 8.17–8.19 (d, 1H, Ar-H, J = 4.5 Hz), 8.26–8.28 (d, 1H, Ar-H, J = 4.5 Hz), 8.46–8.48 (d, 1H, Ar-H, J = 4.5 Hz).
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1777 (asymmetric imide C
N), 1777 (asymmetric imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1723 (symmetric imide C
O stretching), 1723 (symmetric imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1619 (aromatic C
O stretching), 1619 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching), 826 (C–N bending).
C stretching), 826 (C–N bending).![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1773 (asymmetric imide C
N), 1773 (asymmetric imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1722 (symmetric imide C
O stretching), 1722 (symmetric imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1643 (aromatic C
O stretching), 1643 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching), 832 (C–N bending).
C stretching), 832 (C–N bending).2.4. Preparation of the PI films
A solution of the polymer was made by dissolving about 0.4 g of the PI sample in 2 mL of NMP. The homogeneous solution was casted onto a glass substrate and heated in oven at 80 °C for 8 h to remove most of the solvent; then the semi-dried film was further dried in vacuum at 150 °C for 8 h. The obtained films were around 30 μm thick and were used for further characterization.2.5. Equipments
Carbon, hydrogen and nitrogen content of the compounds were determined by pyrolysis method by Vario EL elemental analyzer. 1H-NMR spectra was recorded on a Bruker 500 and 125 MHz instrument (Switzerland) using dimethylsulfoxide (DMSO-d6) as solvent. The FT-IR spectra were taken at room temperature with a resolution of 4 cm−1 using 400 D IR spectrophotometer (Japan). Band intensities are assigned as weak (w), medium (m), strong (s), and broad (br). Inherent viscosity (ηinh) of the polymers in DMAc was measured at about 0.5 g dL−1 concentration with an Ubbelohde viscometer at 30 ± 0.5 °C. Gel permeation chromatography (GPC) was performed with a Waters instrument (Waters 2414) and tetrahydrofuran (THF) was used as eluent (flow rate 0.5 mL min−1). Polystyrene was used as standard and RI detector was used to record the signal in GPC. DSC measurements were made on a DSC Q200 instrument at a heating/cooling rate of 20 °C min−1 under nitrogen. The glass transition temperature (Tg) was taken at the middle of the step transition in the second heating run. Thermal decomposition of these polymers was measured on a TA Instruments thermo gravimetric analyzer, model TGA-2950. A heating rate of 20 °C min−1 was used for determination of the decomposition temperature (Td) under air. Tensile strength and elongation at break of thin PI membranes were measured with the help of UTM-INSTRON, PLUS, model no. 8800. Test samples with dimension of 10 × 25 mm2 and thickness in the range of 0.060 to 0.070 mm were used for the measurement of tensile strength and percentage of elongation at break. The dielectric constants of the PI films were calculated from the capacitance values, measured by the parallel plate capacitor method using HIOKI 3532-50 LCR Hi Tester at 1 MHz at a temperature of 30 °C and a relative humidity of 45. Water absorption of the films were measured using a Mettler microbalance of sensitivity of 10–6 g after immersing the films in double distilled water for 72 h at 30 °C. Ultra violet spectra of the polymer films were recorded at room temperature using Detector SD-2000 (Ocean Optics Inc.) and source lamp DH-2000. The XRD patterns were collected by using a Philips Xpert MPD X-ray diffractometer. The diffractograms were measured for 2θ, in the range of 10–80°, using a voltage of 40 kV and Cu Kα incident beam (λ = 1.51418 Å). The densities of the membranes were measured using Wallace High Precision Densimeter-X22B (UK) using water displacement method. The gas transport properties of the PI membranes were studied at 3.5 bar of applied gas pressure and at 35 °C using automated Diffusion Permeameter (DP-100-A) manufactured by Porous Materials Inc., USA. Ultrahigh pure (99.99%), methane, nitrogen, oxygen, and carbon dioxide gases from BOC Gases, were used for the permeation study. The permeability coefficient, ideal permselectivity (α), diffusion coefficient (D), ideal diffusivity selectivity (αD), solubility coefficient (S), and ideal solubility selectivity (αS) were determined according to reported procedure.22 The reproducibility of the measurements was checked from three independent measurements using the same membrane and it was better than ±5%.2.6. Measurements of gas transport properties
The permeation cell was placed in a thermostatically controlled housing for maintaining isothermal measurement conditions. The effective permeation area (A) was 5.069 cm2. The membranes were degassed for at least 10 h at 35 °C within the permeation cell prior to the gas permeation study. To the upstream side of the membranes, the gas pressure (pi = 3.5 bar) was applied instantaneously and in the downstream side a reservoir of constant volume (119 cm3) was connected with a pressure transducer to observe the total amount of gas which passed through the membranes. The time-lag method was used for the gas transport measurements. This technique allows the determination of mean permeability coefficient (P) from the steady state gas pressure increment (dp/dt)s in the calibrated volume V of the product standard temperature and pressure (T0 = 273.15 K, p0 = 1.026 bar), T is the temperature of measurement, d is the thickness of the membrane and (dp/dt)s was obtained from the slope of the increments of downstream pressure vs. time plot. The reproducibility of the measurements was better than ±5% for CO2 and O2 permeability measurement but the reproducibility was in the range of ±10% for CH4 and N2 permeability measurements. The mean permeability coefficient was determined from eqn (1):
 | (1) |
Permeability coefficient (P) is a product of diffusivity (D) and solubility coefficient (S). The solubility coefficient S was calculated from eqn (2):
 | (2) |
Diffusion coefficient (D) was calculated from the time-lag θ according to eqn (3):
 | (3) |
The ideal permselectivity of the polymer membranes for a pair of gases A and B is the ratio of the individual permeability and can be expressed according to the follows eqn (4):
 | (4) |
3. Results and discussion
3.1. Preparation and characterization of the diamine monomer
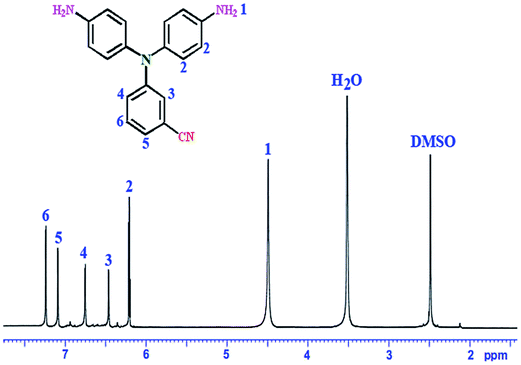
A specific diamine was prepared via a two-step procedure: firstly, nucleophilic substitution reaction of 3-aminobenzonitrile with 1-fluoro-4-nitrobenzene resulted in preparation of 3-(bis(4-nitrophenyl)amino)benzonitrile. Reduction of nitro group in the second step by hydrazine in the presence of Pd/C led to preparation of the diamine compound with benzonitrile pendent group (Scheme 1).The chemical structure of the synthesized diamine was clearly confirmed by FT-IR, 1H-NMR and 13C-NMR spectroscopy and elemental analysis techniques. Good agreement between calculated and found amounts of C, H, and N content was observed in elemental analysis of diamine approving its proposed structure. The FT-IR absorptions related to the nitrile group is appeared at 2225 cm−1. The familiar double absorption peaks of amine functions N–H are obvious around 3448–3377 cm−1. In 1H-NMR spectrum of the diamine compound (Fig. 1), a peak at 4.50 ppm was assigned to the amine protons group and all aromatic protons appeared at 6.25–7.38 ppm. Further confirmation about the structure of diamine was derived from the 13C-NMR spectrum (Fig. 2), where the peak for carbon atom of nitrile group was observed at 112.31 ppm. Also, the peaks for carbon atoms of aromatic rings were observed in the range of 108.22–148.33 ppm. Thus, the NMR data and elemental analysis confirmed that the structure of the diamine agreed with the proposed molecular structure.
3.2. Preparation and characterization of the polymers
Solution polycondensation reactions of the synthesized diamine with different aromatic dianhydrides (6FDA, BTDA, OPDA and PSDA) were performed for preparation of four novel PIs as shown in Scheme 2. The polycondensation reaction was carried out via a two-step procedure: firstly, the diamine was reacted with dianhydride at room temperature to produce PAA. In the second step, resulted PAA was cyclo-imidized and converted to relate PIs. As shown in Table 1, the obtained polymer showed inherent viscosities between 0.97 and 1.13 dL g−1 at a concentration of 0.5 g dL−1. This was further supported by gel permeation chromatography (GPC) measurements which gave Mn, Mw and polydispersities (Mw/Mn) in the range 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 380–77
380–77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 894, 141
894, 141![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–152
000–152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 1.90–2.10, respectively.
000 and 1.90–2.10, respectively.
| Sample | ηinha (dL g−1) | Mnb | Mwc | PDId |
|---|---|---|---|---|
| a Inherent viscosity of the PAAs precursor measured at a concentration of 0.5 g dL−1 in DMAc at 30 °C.b Mn: number average molar mass.c Mw: mass average molar mass.d Polydispersity index: Mw/Mn. | ||||
| PIa | 1.13 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 380 380 |
152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.1 |
| PIb | 0.97 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
141![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.9 |
| PIc | 1.05 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 894 894 |
148![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.9 |
| PId | 1.02 | — | — | — |
3.3. Spectroscopic analysis
Conversion of the PAAs to the fully cyclized PIs was proved by means of by FT-IR, 1H NMR spectroscopy and elemental analysis. All PAAs showed a broad absorption characteristic band of carboxylic O–H and amide N–H groups at about 2600–3700 cm−1 and a narrow characteristic band at 1685 cm−1 associated to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of amide linkage. Also the nitrile group is appeared at 2227 cm−1. Complete imidization reaction of PAAs and formation of PIs were confirmed by FT-IR spectroscopy. The FT-IR spectra of the PIs showed the characteristic absorption bands of the imide absorption at 1777 (C
O of amide linkage. Also the nitrile group is appeared at 2227 cm−1. Complete imidization reaction of PAAs and formation of PIs were confirmed by FT-IR spectroscopy. The FT-IR spectra of the PIs showed the characteristic absorption bands of the imide absorption at 1777 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretching), 1722 (C
O asymmetric stretching), 1722 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretching), 1353 (C–N stretching), and 702 cm−1 (C
O symmetric stretching), 1353 (C–N stretching), and 702 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bending). The disappearance of absorption in the region 2600–3700 cm−1 (O–H and N–H stretch) and around 1685 cm−1 (amide C
O bending). The disappearance of absorption in the region 2600–3700 cm−1 (O–H and N–H stretch) and around 1685 cm−1 (amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch) confirms that PAAs completely conversed into PIs. The FT-IR spectra of the PAA and corresponding PI based on 6FDA and PI based on BTDA are presented in Fig. 3.
O stretch) confirms that PAAs completely conversed into PIs. The FT-IR spectra of the PAA and corresponding PI based on 6FDA and PI based on BTDA are presented in Fig. 3.
Fig. 4 exhibit a typical 1H-NMR spectra of the PI based on 6FDA (a), in which all the peaks have been readily assigned to the hydrogen atoms of the repeating unit and no amide or acid protons at 10–12 ppm appears, indicating that complete imidization was really achieved and PAA film converted into PI. The assignments of each proton designated in the 1H-NMR spectrum are in complete agreement with the proposed polymer structures. The sharp peaks at 4.50 ppm corresponding to the amine protons in 1H-NMR spectrum of diamine disappear completely here, and new peaks appear at 7.50–8.40 ppm which correspond to the protons in the dianhydride units (Fig. 4). All PIs were also recognized by elemental analysis, which were in good agreement with the calculated ones of the proposed structures. These results show that the synthesis of new PIs should be successful and practical in this work.
3.4. Solubility
It is well known that aromatic PIs generally show rather poor solubility in organic solvents in full imidation formation, especially for some PIs derived from rigid dianhydrides such as PMDA. To enhance the solubility, bulky groups, flexible linkages or noncoplanar structures have been introduced along the polymer chains.23–25 It should be noted that good solubility in low-boiling-point solvents is critical for preparing films or coatings at a relatively low processing temperature, which is desirable for advanced microelectronics manufacturing applications. The solubility of the obtaining polymers was examined by dissolving 10 mg of the PIs in 1 mL several organic solvents at ambient temperature or upon heating, as shown in Table 2. It can be seen that all the PIs, can be dissolved in polar solvents, such as NMP, DMF, DMAc, and DMSO, at ambient temperature. The solubility varied depending on the dianhydrides used. PIa based on 6FDA, possessed good solubility owing to the excellent flexibility of hexafluoroisopropylidene structure in 6FDA and could be dissolved readily in DMSO, NMP, DMF, DMAc, m-cresol and THF, at ambient temperature. Also for PIc, the presences of flexible ether chain in the dianhydride unit reduce the rigidity of polymer chain and the solubility was increased. Other PIs also have fairly good solubility. The good solubility of these PIs might be attributed to the presence of the polar nitrile linkage and the polarizability resulting from the nitrogen atom in the side chain.| Polymer | Solubilitya | |||||
|---|---|---|---|---|---|---|
| NMP | DMAc | DMF | DMSO | m-Cresol | THF | |
| a Qualitative solubility was tested with 10 mg of a sample (chemically imidized) in 1 mL of the solvent. + = soluble at room temperature; ± = soluble on heating; − = insoluble. | ||||||
| PIa | ++ | ++ | ++ | ++ | ± | ± |
| PIb | ++ | ++ | ++ | ++ | − | ± |
| PIc | ++ | ++ | ++ | ++ | ++ | ± |
| PId | ++ | ++ | ++ | ++ | − | ± |
3.5. X-ray diffraction
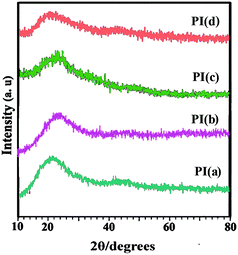
XRD was used to determine the crystalline morphology of the resulting pyridine-containing PIs, and the patterns are shown in Fig. 5. All the XRD patterns of the thermally cured PI films showed a diffuse characteristic diffraction peak at around 2θ = 16–30° and no distinct crystal peaks, these should be the evidences that the PIs held completely amorphous structures. Furthermore, the amorphous character is also supported by the reasonable solubility of PIs, which is in agreement with the general rule that the solubility decreases with increasing crystallinity.3.6. Mechanical properties of PI films
PI films were subjected to tensile testing and their stress–strain curves were shown in Fig. 6. Table 3 exhibits the mechanical properties of the PIs, including the tensile strength, tensile modulus, and elongations to break. These films had tensile strength of 90–115 MPa, elongations at breakage of 13–20%, and tensile moduli of 1.29–1.68 GPa which shows strong and hard materials. PIa derived from the aromatic dianhydride 6FDA, shows small tensile strength of 95 MPa which could be attributed to the rigid structure of polymer backbone.| Polymer | Modulusa (GPa) | Ultimate strengthb (MPa) | Ultimate elongationc (%) | Water absorption at 30 °C (%) | Dielectric constant at 30 °C (1 MHz) |
|---|---|---|---|---|---|
| a Initial slope of the stress strain curve.b Stress at break.c Elongation at break. | |||||
| PI(a) | 1.29 | 95 | 17 | 0.32 | 1.98 |
| PI(b) | 1.43 | 102 | 15 | 0.56 | 2.23 |
| PI(c) | 1.68 | 115 | 13 | 0.88 | 2.14 |
| PI(d) | 1.34 | 90 | 20 | 0.43 | 2.38 |
3.7. Water absorption and dielectric properties
Water absorption is a great concern when the polymers are used in electronic packaging as dielectric insulation.26,27 The absorbed water in the polymer structure affects their dielectric performance. Hence, for materials designed for such type of applications the water absorption data is an essential parameter. The water absorption of the PIs membranes was measured at 30 °C (Table 3). The water absorption values for these PIs were between 0.32 to 0.88%. PIa showed lowest water absorption among all the synthesized polymers due to the presence of hydrophobic –CF3 groups in the anhydride moiety which is similar to the previous study.28,29The dielectric constant of PI membrane was measured by using a capacitance meter (1 MHz at 30 °C, RH 45%). The dielectric constant and the dissipation factor are the important properties to determine the use of a material in interlayer dielectrics. The dielectric constant values of the PI membranes were presented in Table 3. In general, the PIs exhibited low dielectric constant values. This should be attributed to the incorporation of bulky benzonitrile substituents. The increasing order of dielectric constant followed the order as PId > PIb > PIc > PIa. The lower dielectric constant values may be attributed to the presence of bulky –CF3 groups which hinders close interchain packing and to the low polarizability of the C–F bonds.28–30 Among the PI films, PIa showed the lowest dielectric constant (1.98) and had a decrease of 1.5 compared to that of the commercial Kapton films (3.48). This can be explained by the additional of fluorine moiety.
3.8. Thermal properties
The thermal properties of the benzonitrile-containing polymers were evaluated by TGA at the heating rate of 20 °C min−1 from 20 to 800 °C under N2 flow. Fig. 7 shows the TGA curves of the synthesized PIs, and the thermal behavior parameters are listed in Table 4. The obtained PIs did not exhibit obvious weight loss before 365 °C, indicating that no thermal decomposition had occurred. As shown in Fig. 7, the decomposition-starting temperatures were in the range of 366–400 °C. The 5 and 10% weight loss temperatures (T5% and T10%) were in the ranges of 366–403 °C and 400–420 °C, respectively. The results showed that PIa, prepared from 6FDA, showed the best thermal stability. The amount of carbonized residue (char yield or CR) of PIs was from 66 to 72% at 800 °C under a nitrogen gas flow, depending on the structure of dianhydride component. The high char yields of these polymers can be attributed to their high aromatic content.| Entry | Material | T5a (°C) | T10a (°C) | Char yieldb (%) | Tgc (°C) |
|---|---|---|---|---|---|
| a Temperature at which 5 and 10% weight loss was recorded by TGA at a heating rate of 20 °C min−1 in a nitrogen atmosphere.b Percentage weight of material left undecomposed after TGA analysis at maximum temperature 800 °C in a nitrogen atmosphere.c Measured at a heating rate of 20 °C min−1 under N2 atmosphere. | |||||
| 1 | PIa | 403 | 420 | 72 | 299 |
| 2 | PIb | 402 | 418 | 70 | 296 |
| 3 | PIc | 400 | 412 | 69 | 295 |
| 4 | PId | 366 | 400 | 66 | 291 |
DSC was applied to determine glass transition temperature (Tg) of the PIs. As seen from Table 4, all the PIs exhibited Tg values higher than 291 °C, possibly because the films have high stiffness, which is mainly ascribed to the structure of the repeating units, stiffness of the polymer backbones and polymer intermolecular forces. As the diamine used for preparing the PIs was completely similar, the different Tg values resulted mainly from the different rigidities of the dianhydrides. For PIa, CF3 groups may have led to increased chain rigidity, which consequently led to a much higher Tg.
3.9. Optical transparency
Thin films of PIs (a–b) were measured for optical transparency with UV-vis spectroscopy. Fig. 8 exhibits the UV-vis transmittance spectra of the PI films. The cut-off wavelength (absorption edge, λ0) values and the percentage of transmittance at 500 nm from these spectra were listed in Table 5. The most polymers between the UV and visible area show strong absorption due to the highly conjugated aromatic structures and intermolecular charge-transfer complex formation of PI.31 The cut-off wavelength value (352–362 nm) and the percentage of transmittance (80–83%) at 500 nm for these PI membranes are tabulated in Table 5. The trifluoro methyl groups and flexible ether linkages in the dianhydrides moieties are very much effective in decreasing charge transfer complex formation and improve the optical properties of the PIs.29,31,32 The PIa showed highest optical transparency this is due to the presence of trifluoro methyl groups in anhydride part.3.10. Gas separation performance test
Gas transport properties of four different gases (CO2, O2, N2, CH4) through these newly synthesized aromatic PIs were studied at 35 °C under an upstream pressure of 3.5 bar. It is well known that permeability coefficient varies depending on various casting protocols adopted in different laboratories. However, the ideal permselectivity value for a pair of gas can be considered as a standard parameter to compare gas separation performance of a series of polymeric membranes with other polymeric membranes measured in other laboratories.33 Gas permeability coefficients and permselectivity values have been presented in Table 6. The diffusion coefficients and solubility coefficient values are shown in Table 7. The solubility selectivity and diffusivity selectivity values have been also depicted in Table 7. Since we could not carry out direct sorption measurements for these four polymeric membranes due to limited equipment, definitely, a direct sorption measurement could provide a better insight on the permeation mechanism and more reliable diffusion coefficient values. The diffusion coefficients were determined using the time-lag value of gas flow vs. time plot. We understand that the diffusion coefficient values determined from time-lag values are unreliable when the dual mode transport characteristics apply but within a series of polymers the measured values of diffusion coefficients can be used for the comparative study of their general behavior.34| Film | P(O2) | P(N2) | P(CO2) | P(CH4) | P(O2)/P(N2) | P(CO2)/P(CH4) | P(CO2)/P(O2) | P(CO2)/P(N2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a Permeability coefficient (P) in Barrer (1 Barrer) 1 cm3 (STP) cm cm−2 s−1 cmHg−1 × 10−10, measured at 35 °C. | |||||||||
| PIa | 7.05 | 0.84 | 16.77 | 0.22 | 8.40 | 76.22 | 2.38 | 19.96 | — |
| PIb | 2.14 | 0.55 | 7.76 | 0.31 | 3.90 | 25.03 | 3.63 | 14.11 | — |
| PIc | 1.12 | 0.34 | 3.76 | 0.21 | 3.29 | 17.90 | 3.36 | 11.06 | — |
| PId | 3.33 | 0.44 | 10.08 | 0.76 | 7.57 | 13.26 | 3.03 | 22.91 | — |
| Kapton | 0.64 | 0.11 | 3.51 | 0.06 | 5.82 | 58.50 | 5.48 | 31.91 | 2 |
| Polymer | CO2 | O2 | (CO2)/(CH4) | (O2)/(N2) | ||||
|---|---|---|---|---|---|---|---|---|
| D | S | D | S | αDa | αSa | αD | αS | |
| a αD = diffusivity selectivity values of gas pairs (DA/DB). αS = solubility selectivity values of gas pairs (SA/SB). | ||||||||
| PIa | 2.98 | 8.54 | 8.22 | 1.98 | 2.58 | 10.65 | 1.94 | 0.64 |
| PIb | 1.44 | 2.93 | 4.76 | 0.95 | 1.93 | 7.54 | 1.15 | 0.78 |
| PIc | 0.88 | 2.22 | 2.99 | 0.66 | 0.38 | 8.92 | 0.42 | 1.34 |
| PId | 1.88 | 4.96 | 5.86 | 0.94 | 1.08 | 10.12 | 0.92 | 1.55 |
Fractional free volumes (FFVs) for all the PI membranes with respect to four different gases were estimated using group contribution method developed by Park and Paul.33 The order of fractional free volume for the PI membranes for all the gases was PIa > PId > PIb > PIc (Table 8). Incorporation of hexafluro linkage in the main chain is known to render loose chain packing consequently PIa have higher fractional free volume in the series.36 The order of gas permeability coefficients of four PI membranes for all the gases was PIa > PId > PIb > PIc. This decreasing trend of permeability coefficient of these PIs (from ‘PIa’ to ‘PId’) nicely correlates with their corresponding FFV values for all the gases.35 The dependence of permeability coefficients with FFV of the PIs for the four gases (CO2, O2, N2 and CH4) is represented in Table 8.
| Polymer | Densitya | (FFVb)CH4 | (FFVb)N2 | (FFVb)O2 | (FFVb)CO2 |
|---|---|---|---|---|---|
| a Density (g cm−3) measured at 30 °C.b FFV = [V − (Vo)n]/V, where V is the volume per mole of the repeat unit of the polymer at 30 °C, (Vo)n = ∑γnk(Vw)k, γnk is set of empirical factors depending upon gas ‘n’ and group ‘k’. (Vw)k represents van der Waals volumes for group ‘k’.35 | |||||
| PIa | 1.08 | 0.21 | 0.23 | 0.25 | 0.22 |
| PIb | 1.52 | 0.10 | 0.12 | 0.11 | 0.11 |
| PIc | 1.32 | 0.14 | 0.13 | 0.11 | 0.12 |
| PId | 1.38 | 0.14 | 0.17 | 0.19 | 0.16 |
For amorphous polymers the logarithm of permeability is found to decrease almost linearly with the increasing reciprocal fractional free volume.36 All the synthesized PIs follow the above trend. For all the PIs, the gas permeability coefficient follow the order as P(CO2) >P(O2) > P(N2) > P(CH4) which is basically the reverse order of their kinetic diameter (Å): CO2 (3.3) < O2 (3.46) < N2 (3.64) < CH4 (3.8). The order of diffusion coefficient for the PIs followed the order as D (O2) > D (CO2) > D (N2) > D (CH4). A different order of diffusion coefficients compared to the permeability coefficients order for these four gases can be explained by the fact that there is no or little interaction between O2, CH4, and N2 with the PIs. However, there is some sort of interaction between CO2 and PIs. The interaction is due to the fact that PI has imide linkage in chain repeating unit. Permeability of CO2 through each PIs was higher than the other gases due to the higher solubility coefficient values of CO2 for each PIs.37 From Tables 6 and 7 it can be interpreted that ideal permselectivities for CO2/O2, CO2/N2 and CO2/CH4 gas pairs are mainly due to the solubility selectivities as diffusivity selectivities are reasonably small.38
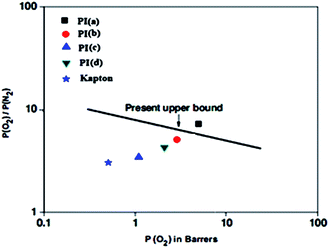
The ideal gas separation performances of this polymeric membrane have been compared with commercially available polymer membrane (Kapton). For a better comparison, the gas separation performances of these PIs in comparison to the other polymers have been presented as Robeson plots20,21 of permselectivity of O2/N2 gas pair vs. permeability of O2 (Fig. 9) and permselectivity of CO2/CH4 gas pair vs. permeability of CO2 (Fig. 10). In comparison to Kapton, these polymers showed excellent permeability with better separation performances for both O2/N2 and CO2/CH4 gas pairs. Especially, PIa showed higher permeability as well as higher permselectivity for CO2/CH4 and O2/N2 gas pairs in comparison to the Kapton and PIb surpassed the upper boundary limit drawn by Robeson for O2/N2 gas pair.20,21
4. Conclusion
A series of processable PIs with benzonitrile pendant group were synthesized from a diamine monomer, 3-(bis(4-aminophenyl)amino)benzonitrile with four different aromatic dianhydrides via thermal or chemical imidization method. The resulting polymers showed good solubility in polar amidic solvents, excellent thermal stability, and high glass transition temperatures. The solutions of intermediate PAA could be cast into flexible films with good mechanical properties and optical transparency, and low dielectric constants. Transparent and flexible membranes with very good thermal and mechanical properties made PIs suitable candidates for membrane based gas permeation study. Gas transport properties of CO2, O2, N2, and CH4 through these newly synthesized PI membranes were successfully. All the PIs showed almost similar permeability coefficients with different permselectivity values except PIb. PIa exhibited very high permeability for all the gases with higher permselectivity for different gas pairs and surpassed the upper boundary limit drawn by Robeson for O2/N2 gas pair. Dielectric properties for these PIs were determined and used to correlate with their permeability coefficients. The incorporation of benzonitrile units into the polymer backbone leads to soluble polymeric materials that are amenable for use as high-performance materials and demonstrate a promising potential for future application.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
We wish to express our gratitude to the Research Affairs Division Isfahan University of Technology (IUT), Isfahan, for partial financial support. Further financial support from National Elite Foundation (NEF), and Iran Nanotechnology Initiative Council (INIC) is gratefully acknowledged.References
- M. K. Ghosh and K. L. Mittal, Polyimides: Fundamentals and Applications, Marcel Dekker, New York, 1996 Search PubMed
.
- Q. H. Zhang, W. Q. Luo, L. X. Gao, D. J. Chen and M. X. Ding, Thermal Mechanical and Dynamic Mechanical Property Of Biphenyl Polyimide Fibers, J. Appl. Polym. Sci., 2004, 92, 1653–1657 CrossRef CAS
.
- S. J. Zhang, Y. F. Li, D. X. Yin, X. L. Wang, X. Zhao, Y. Shao and S. Y. Yang, Study on Synthesis and Characterization of Novel Polyimides Derived from 2,6-Bis(3-aminobenzoyl)pyridine, Eur. Polym. J., 2005, 41, 1097–1107 CrossRef CAS PubMed
.
- S. Mallakpour and M. Dinari, In Situ Fabrication of High performance Polyimide/Tyrosine-Modified Layered Silicate Nanocomposites, Nanotechnology, 2012, 7(1250021), 1–10 Search PubMed
.
- P. M. Hergenrother, K. A. Watson, J. G. Smith, J. W. Connell and R. Yokota, Polyimides from 2,3,3′,4′-Biphenyltetracarboxylic Dianhydride and Aromatic Diamines, Polymer, 2002, 43, 5077–5093 CrossRef CAS
.
- R. Verker, N. Atar, F. Quero, S. J. Eichhorn and E. Grossman, Tensile Stress Effect on the Macromolecular Orientation and Erosion Mechanism of an Atomic Oxygen Irradiated Polyimide, Polym. Degrad. Stab., 2013, 98, 997–1005 CrossRef CAS PubMed
.
- J. Y. Kong, M. C. Choi, G. Y. Kim, J. J. Park, M. Selvaraj, M. J. Han and C. S. Ha, Preparation and Properties Of Polyimide/Grapheme Oxide Nanocomposite Films With Mg Ion Crosslinker, Eur. Polym. J., 2012, 48, 1394–1405 CrossRef CAS PubMed
.
- M. Dinari and H. Ahmadizadegan, Preparation, Characterization and Gas Separation Properties of Nanocomposite Materials Based on Novel Silane Functionalizing Polyimide Bearing Pendent Naphthyl Units and ZnO Nanoparticles, RSC Adv., 2015, 5, 8630–8639 RSC
.
- D. Chao, S. Wang, R. Yang, E. B. Berda and C. Wang, Synthesis and Properties of Multifunctional Poly(Amic Acid) With Oligoaniline and Fluorene Groups, Colloid Polym. Sci., 2013, 291, 2631–2637 CAS
.
- M. Saleh, H. M. Lee, K. C. Kemp and K. S. Kim, Highly Stable CO2/N2 and CO2/CH4 Selectivity in Hyper-Cross-Linked Heterocyclic Porous Polymers, ACS Appl. Mater. Interfaces, 2014, 6, 7325–7333 CAS
.
- G. Waris, H. M. Siddiqi, M. Bolte, R. Hossain and Z. Akhtar, Synthesis and Characterization of Processable Aromatic Polyimides and Their Initial Evaluation as Promising Biomaterials, Colloid Polym. Sci., 2013, 291, 1581–1593 CAS
.
- M. K. Madhra, A. K. Salunke, S. Banerjee and S. Prabha, Synthesis and Properties of Fluorinated Polyimides Derived from Novel 2,6-Bis(3′-trifluoromethyl-p-aminobiphenyl ether) Pyridine and 2,5-Bis(3′-trifluoromethyl-p-aminobiphenyl ether)thiophene, Macromol. Chem. Phys., 2002, 203, 1238–1248 CrossRef CAS
.
- S. Mallakpour and M. Dinari, Fabrication of Polyimide/Titania Nanocomposites Containing Benzimidazole Side Groups via Sol–Gel Process, Prog. Org. Coat., 2012, 75, 373–378 CrossRef CAS PubMed
.
- M. Bruma, F. Mercer, B. Schulz, R. Dietel, J. Fitch and P. Cassidy, Study of the Crosslinking Process in Fluorinated Poly(Imide-Amide)s Containing Pendant Cyano Groups, High Perform. Polym., 1994, 6, 183–191 CAS
.
- E. Hamciuc, M. Bruma, B. Schulz and T. Kopnick, New Silicon-Containing Poly(imide-amide)s, High Perform. Polym., 2003, 15, 347–359 CrossRef CAS PubMed
.
- C. G. Wang, Y. Koyama, S. Uchida and T. Takata, Synthesis of Highly Reactive Polymer Nitrile N-Oxides for Effective Solvent-Free Grafting, ACS Macro Lett., 2014, 3, 286–290 CrossRef CAS
.
- B. Lin and X. Xu, Preparation and Properties of Cyano-Containing Polyimide Films Based on 2,6-Bis(4-aminophenoxy)benzonitrile, Polym. Bull., 2007, 59, 243–250 CrossRef CAS
.
- K. Ghosal and B. D. Freeman, Gas Separation Using Polymer Membranes: An Overview, Polym. Adv. Technol., 1994, 5, 673–697 CrossRef CAS
.
- W. J. Koros and M. W. Hellums, Gas Separation Membrane Material Selection Criteria: Differences For Weakly And Strongly Interacting Feed Components, Fluid Phase Equilib., 1989, 53, 339–354 CrossRef CAS
.
- L. M. Robeson, Correlation of Separation Factor versus Permeability for Polymeric Membranes, J. Membr. Sci., 1991, 62, 165–185 CrossRef CAS
.
- L. M. Robeson, The Upper Bound Revisited, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS PubMed
.
- S. K. Sen and S. Banerjee, High Tg, Processable Fluorinated Polyimides Containing Benzoisoindoledione Unit and Evaluation of their Gas Transport Properties, RSC Adv., 2012, 2, 6274–6289 RSC
.
- A. Ghosh, S. K. Sen, S. Banerjee and B. Voit, Solubility Improvements in Aromatic Polyimides by Macromolecular Engineering, RSC Adv., 2012, 2, 5900–5926 RSC
.
- Y. T. Chern, J. T. Twu and J. C. Chen, High Tg and High Organosolubility of Novel Polyimides Containing Twisted Structures Derived from 4-(4-Amino-2-chlorophenyl)-1-(4-aminophenoxy)-2,6-di-tert-butylbenzene, Eur. Polym. J., 2009, 45, 1127–1138 CrossRef CAS PubMed
.
- S. Mallakpour and M. Dinari, High Performance Polymers in Ionic Liquid: A Review on Prospects for Green Polymer Chemistry. Part II: Polyimides and Polyesters, Iran. Polym. J., 2011, 20, 259–279 CAS
.
- Polyimides: Fundamentals and Application, ed. Ghosh M. K. and Mittal K. L., Marcel Dekker, New York, 1996 Search PubMed
.
- Kirk-Othmer Encyclopedia of Chemical Technology, ed. Takekoshi T., Wiley, New York, 1996, vol. 19, pp. 813–837 Search PubMed
.
- L. Tao, H. Yang, J. Liu, L. Fan and S. Yang, Synthesis and Characterization of Highly Optical Transparent and Low Dielectric Constant Fluorinated Polyimides, Polymer, 2009, 50, 6009–6018 CrossRef CAS PubMed
.
- W. Jang, D. Shin, S. Choi, S. Park and H. Han, Effects of Internal Linkage Groups of Fluorinated Diamine on the Optical and Dielectric Properties of Polyimide Thin Films, Polymer, 2007, 48, 2130–2143 CrossRef CAS PubMed
.
- C. Wang, W. Chen, Y. Chen, X. Zhao, J. Li and Q. Ren, Synthesis and Properties of New Fluorene-based Polyimides Containing Trifluoromethyl and Isopropyl Substituents, Mater. Chem. Phys., 2014, 144, 553–559 CrossRef CAS PubMed
.
- C. P. Yang, S. H. Hsiao and K. H. Chen, Organosoluble and Optically Transparent Fluorine-Containing Polyimides Based on 4,4′-Bis(4-amino-2-trifluoromethylphenoxy)-3,3′,5,5′-tetramethylbiphenyl, Polymer, 2002, 43, 5095–5104 CrossRef CAS
.
- V. Kute and S. Banerjee, Polyimides 7: Synthesis, Characterization, and Properties of Novel Soluble Semifluorinated poly(ether imide)s, J. Appl. Polym. Sci., 2007, 103, 3025–3044 CrossRef CAS
.
- J. Y. Park and D. R. Paul, Correlation and Prediction of Gas Permeability in Glassy Polymer Membrane Materials via a Modified Free Volume Based Group Contribution Method, J. Membr. Sci., 1997, 125, 23–39 CrossRef CAS
.
- M. B. Moe, W. J. Koros, H. H. Hoehn and G. R. Husk, Effects of Film History on Gas Transport in a Fluorinated Aromatic Polyimide, J. Appl. Polym. Sci., 1988, 36, 1833–1846 CrossRef CAS
.
- D. R. Paul, Gas Sorption and Transport in Glassy Polymers, Ber. Bunsenges. Phys. Chem., 1979, 83, 294–302 CrossRef CAS
.
- M. Dinari and H. Ahmadizadegan, Synthesis, Structural Characterization and Properties of Novel Functional Poly(ether imide)/Titania Nanocomposite Thin Films, Polymer, 2014, 55, 6252–6260 CrossRef CAS PubMed
.
- W. M. Lee, Selection of Barrier Materials from Molecular Structure, Polym. Eng. Sci., 1980, 20, 65–69 CAS
.
- M. D. Guiver, G. P. Robertson, Y. Dai, F. Bilodeau, Y. S. Kang, K. J. Lee, J. Y. Jho and J. Won, Structural Characterization and Gas-transport Properties of Brominated Matrimid Polyamide, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 4193–4204 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2015 |