Hierarchical three-dimensional NiCo2O4 nanoneedle arrays supported on Ni foam for high-performance supercapacitors†
Abstract
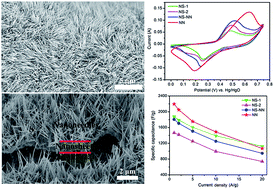
Three-dimensional (3D) hierarchical NiCo2O4 nanoneedle arrays have been prepared on nickel foam via a facile hydrothermal method followed by annealing in air. Impressively, when investigated as binder-free supercapacitor electrodes, such unique NiCo2O4 nanoneedle arrays on Ni foam exhibit a superior specific capacitance of 2193 F g−1 and 1490 F g−1 at current densities of 1 and 10 A g−1 calculated based on the active mass of NiCo2O4, respectively. Furthermore, the areal capacitance is 3.71 F cm−2 at 1 mA cm−2 and 1.39 F cm−2 at 40 mA cm−2. The remarkable electrochemical performance is due to the hierarchical nanoneedle array structure with bottom crosslinked nanosheets, which has a large surface area, thus providing more sites to facilitate electrochemical reactions, rapid ion/electron transport, and enhanced strain accommodation. Our results demonstrate that the hierarchical NiCo2O4 nanoneedle arrays are a promising material as a binder-free electrode for high performance supercapacitors.

 Please wait while we load your content...
Please wait while we load your content...