Synthesis of an amphiphilic hyperbranched polymer as a novel pH-sensitive drug carrier†
Rui Chenac and
Liqun Wang*ab
aDepartment of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, Chinan. E-mail: lqwang@zju.edu.cn
bMOE Key Laboratory of Macromolecular Synthesis and Functionalization, Hangzhou 310027, China
cSchool of Chemistry and Chemical Engineering, State Key Laboratory of Metal Composites, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, China
First published on 21st January 2015
Abstract
An amphiphilic hyperbranched polymer containing large amounts of pH sensitive bonds was synthesized by our group. The hydrophobic chains are hyperbranched polyacetals (HBPAs) and the hydrophilic chains are polyethylene glycols. Polyethylene glycols are attached to the hyperbranched polyacetals by the hydrazone bonds. The amphiphilic hyperbranched polymer could be assembled into micelles easily by the dialysis method. The micelles containing large amounts of pH sensitive bonds were quite fragile in pH 5.0 buffer solution but very stable in pH 7.4 buffer solution. DOX-loaded micelles were also prepared by the dialysis method. The sizes of the blanked micelle and DOX-loaded micelle were 30 nm and 35 nm, respectively. The increase of the diameter confirmed that DOX was successfully loaded into the micelle. Drug loading content and drug loading efficiencies were 2.34% and 23.4%, respectively, which was detected by the UV-VIS at the wavelength of 482 nm. The drug release behavior demonstrated that DOX was released faster in pH 5.0 buffer solution than in pH 7.4 buffer solution.
Introduction
Hyperbranched polymers are highly branched macromolecules with three-dimensional dentritic architectures. Due to their unique physical and chemical properties as well as potential applications in various fields from drug delivery to coatings, the interest in hyperbranched polymers is growing rapidly.1–5Compared to linear polymers, they have a number of beneficial attributes for biomedical applications, including the following: (1) biodistribution and pharmacokinetic properties that can be tuned by controlling dendrimer size and conformation; (2) high structural and chemical homogeneity; (3) ability to be functionalized with multiple copies of drugs, chromophores or ligands either at their peripheries and/or their interiors; (4) high ligand density; (5) controlled degradation.6 Due to the above advantages, many hyperbranched polymers, such as PAMAM,7–9 polyether,10 polyester11 and polypeptide12–14 have been designed as drug delivery systems for cancer therapy.
However, for cancer therapy, it is better to bestow the carrier with some response characteristics, such as pH, redox, and temperature responsiveness. Jean M. J. Frechet and cowokers10 synthesized hyperbranched polyesters, which was used as drug carrier. In this system, DOX was covalently bound via a hydrazone linkage to a high molecular weight 3-arm poly(ethylene oxide)–dendrimer hybrid. The drug release was a function of pH, and the release rate was more rapid at pH < 6. K. Haba and coworkers15 designed a dendritic pro-drug that is activated through a single catalytic reaction by a specific enzyme. It could offer significant advantages in the inhibition of tumor growth, especially if the targeted or secreted enzyme exists at relatively low levels in the malignant tissue. R. A. Shenoi and co-workers16 synthesized a new class of biodegradable polymers, main chain biodegradable hyperbranched polyglycerols with randomly distributed acid-labile ketal groups in the medium high molecular weight range. In vitro degradation studies showed that this polymer was relatively stable at physiological pH, but underwent pH dependent hydrolysis at acidic pH values.
Among all of the hyperbranched polymers, we have not heard of a hyperbranched polymer containing both acetal and hydrazone bonds in the backbone of the macromolecule. In our group, we synthesized an amphiphilic hyperbranched polymer, which contains dual pH responsive bonds: acetal bond and hydrazone bond. The hyperbranched core, which consists of acetal linkages, is surrounded by the polyethylene glycol chains, and the polyethylene glycol chains are linked to the core by the hydrazone bond. This new type of amphiphilic hyperbranched polymers are quite pH sensitive, which can be used as drug carriers in the future.
Experimental
Materials
Mono-methoxy polyethylene glycol (PEG, Mw: 1900), 4-(2-hydroxyethoxy)-benzaldehyde were purchased from Tokyo Chemical Industry Co., Ltd. Trimethyl orthoformate, (±)-camphor-10-sulfonic acid, montmorillonite K10 and benzaldehyde dimethyl acetal were purchased from Alfa Aesar Co., Ltd. 4-Nitrophenyl carbonochloridate (p-NPC) was purchased from Adamas Reagent Co., Ltd. Hydrazine monohydrate was purchased from Aladdin Reagent Co., Ltd. Triethylamine, methylene chloride, methanol and n-hexane were purchased from Sinopharm Chemical Reagent Co., Ltd. All the reagents were used as received.Synthesis and preparation
(a) The synthesis of the monomer: 4-(2-hydroxyethoxy)-benzaldehyde dimethyl acetal. 5 g of 4-(2-hydroxyethoxy)-benzaldehyde, 50 mg of montmorillonite K10 (catalyst), 30 ml of trimethyl orthoformate and 100 ml of methanol were added into a 250 ml flask and refluxed at 100 °C with magnetic stirring for 24 h. After the reaction, the catalyst was removed by vacuum filtration and the solvents (methanol and trimethylorthoformate) were removed by rotary evaporation. Then the monomer 4-(2-hydroxyethoxy)-benzaldehyde dimethyl acetal was acquired. (The H1-NMR characterization was in ESI, Fig. 2†).
(b) The synthesis of hyperbranched polyacetals (HBPAs). 1.7 g of 4-(2-hydroxyethoxy)-benzaldehyde dimethyl acetal, 0.08 g of PCS19 and 20 μl of benzaldehyde dimethyl acetal were added in a 10 ml of reaction tube. The mixture was stirred for 15 min under Ar atmosphere at room temperature and the stirring was continued at 70 °C for another 15 min. Then, the temperature was increased to 110 °C slowly and the stirring continued for 1 hour under Ar atmosphere. 1 hour later, the reaction was cooled to room temperature and then heated to 110 °C again under vacuum (<−0.1 MPa) for another 1 h. After the reaction cooled to room temperature, 5 ml of THF was added to dissolve the crude product and then the catalyst PCS was removed by vacuum filtration. THF was removed by rotary evaporation and HBPAs was acquired.
(c) The synthesis of HBPAs–hydrazone–PEG. 180 mg of HBPAs and 2.75 g of pre-synthesized mono-methoxy PEG–NHNH220,21 (see ESI†) were dissolved in 10 ml of DMF. 20 μl of triethylamine was added into the solution. The reaction was kept at room temperature and stirred for 24 hours. 24 hours later, the solution was dialyzed against deionized water for another 24 hours (molecular weight cut off: 3500). The final amphiphilic polymer HBPAs–hydrazone–PEG was obtained by freeze-drying.
(a) The degradation of HBPAs. 5 mg of HBPAs was dissolved into 5 ml of DMF, and dialyzed against 50 ml of buffer solution (molecular cutoff: 100–500). At the predetermined time, 4 ml of buffer solution was withdrawn and 4 ml of fresh buffer solution was then added. 20 μl of collected buffer solution was injected into the HPLC system to detect the hydrolyzed product 4-(2-hydroxyethoxy)-benzaldehyde. The accumulation of the hydrolyzed 4-(2-hydroxyethoxy)-benzaldehyde was calculated as a function of the time.
(b) The degradation of the HBPAs–hydrazone–PEG micelle. 1 mg ml−1 HBPAs–hydrazone–PEG micelle was diluted by the same volume of buffer solution. 30 min later, the diluted micelle was detected by the DLS instrument for size analysis.
Methods
1H-NMR spectroscopy (ADVANCE2B/400 MHz) was used with DMSO-d6 and 1,2-dichlorobenzen d4 as the solvent. Transmission electron microscopy (JEM-1200EX, 80 kV) was performed to investigate the morphology of the HBPAs–hydrazone–PEG micelles and drug-loaded micelles. Size distribution analysis was studied using dynamic light scattering (Malven). Two instruments were used to crosscheck the reliability of the obtained data. The amount of released DOX was measured by a UV detector (UV-1800, SHIMADZU) at a wavelength of 482 nm. Molecular weight was detected by the GPC (Waters 1515).Result and discussion
The synthesis of the amphiphilic hyperbranched polymers
Fig. 1 gives the synthesis route of HBPAs–hydrazone–PEG. The monomer 4-(2-hydroxyethoxyl)-benzaldehyde dimethyl acetal was firstly synthesized by aldol condensation. The transfer efficiency from 4-(2-hydroxyethoxyl)-benzaldehyde to 4-(2-hydroxyethoxyl)-benzaldehyde dimethyl acetal is close to 100%, which was confirmed by the H1-NMR spectrum (ESI, Fig. 2†). Hyperbranched polyacetals were then synthesized from the monomer at the ratio of monomer to initiator (benzaldehyde dimethyl acetal) of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. By changing the monomer to initiator ratio, we could synthesize HBPAs with different molecular weights.
1. By changing the monomer to initiator ratio, we could synthesize HBPAs with different molecular weights.
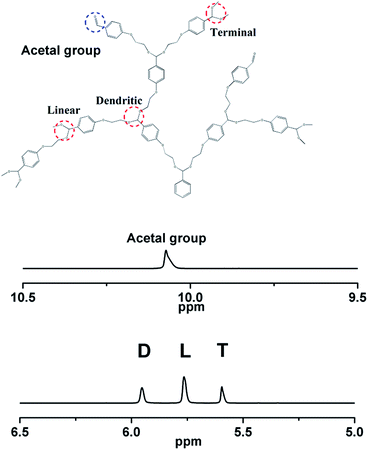
The proton NMR spectra of the polymer HBPAs along with their peak assignments are shown in Fig. 2 (upper). Compared to the monomer spectrum (ESI, Fig. 2†), the peaks in the polymer spectrum become broad, and a substantial reduction in the relative intensity of the methoxy protons (peak g), because of the expected loss of methanol. Interestingly, the methane proton of the acetal unit (peak a), which is a singlet in the monomer, splits into three well-resolved peaks in the polymer. On the basis of the relative positions, these peaks can be assigned into three well-resolved ones. The peaks can be assigned to dendritic (D), linear (L) and terminal (T) units, as shown in Fig. 3. From the relative intensities of these three peaks, the degree of branching (DB) of the polymer was estimated to be around 0.52, which is roughly the expected value for a statistically random growth process.22 The DB was calculated by the following eqn (1):
 | (1) |
In Fig. 2, another interesting aspect of the spectrum is the presence of an aldehyde proton peak at around 10.2 ppm. This is presumably is due to the inadvertent hydrolysis of some of the acetal groups. The relative intensities of the three acetal methane peaks (peaks a) appear to suggest that the preferential hydrolysis of the terminal dimethylacetal (T) units may have occurred; this is reflected by the decrease in the intensity of the terminal methane peak with increase in the intensity of the aldehyde peak (the H1-NMR spectrum is in ESI, Fig. 3†).17 The aldehyde group content could, of course, be adjusted by adding 4-(2-hydroxyethoxy)-benzaldehyde into the monomer in the synthesis procedure of HBPAs (ESI†). Compared to acetal bonds (5.5–6.2 ppm), the content of the aldehyde group (10.2 ppm) was about 17%, which was estimated by the H1-NMR spectrum.
It is the presence of the aldehyde group that makes the reaction between HBPAs and PEG–NHNH2 happen. The amount of PEG–NHNH2 was 10 fold higher than that of the aldehyde group of HBPAs by mole ratio, so that the aldehyde group was transferred into hydrazone bond completely. The proton NMR spectra of the polymer HBPAs–hydrazone–PEG along with their peak assignments are shown in Fig. 2 (bottom). HBPAs–hydrazone–PEG could not dissolve into DMSO very well, but could dissolve into 1,2-dichlorobenzen. This is the reason why we used 1,2-dichlorobenzen d4 as the deuterated solvent. PEG was successfully attached to the HBPAs, which was confirmed by the disappearance of the peak at 10.2 ppm (aldehyde proton peak) and the appearance of the peak at 8.9 ppm (the hydrazone proton peak). The number average molecular weight of HBPAs and HBPAs–hydrazone–PEG was 3600 and 8600, respectively (GPC). The molecular weight could be controlled by the molar ratio of monomer to initiator.
Fig. 4 shows the hydrolysis behavior of HBPAs in the dialysis method. HBPAs was first dissolved into DMF solvent, and then dialyzed against the buffer solution with different pH. At a pre-determined time, 4 ml of buffer solution was withdrawn and 4 ml fresh buffer solution was added into the dialysis system. 20 μl of the collected buffer solution was injected into the HPLC system. 4-(2-Hydroxyethoxyl)-benzaldehyde, which was water soluble, was produced from the hydrolysis of HBPAs and was detected by the UV-VIS system. The accumulated amount of the 4-(2-hydroxyethoxyl)-benzaldehyde was calculated and functioned to the time, as shown in Fig. 4. HBPAs were hydrolyzed faster in pH 5.0 buffer solution than in pH 7.4, which demonstrates that HBPAs were pH sensitive.
The micelle formation of HBPAs–hydrazone–PEG
Micelles were prepared by dissolving HBPAs–hydrazone–PEG in DMF and dialyzed in the deionized water for 24 h. Fig. 5 gives the DLS curve and TEM of the prepared HBPAs–hydrazone–PEG micelle. The diameter of the micelle is about 30 nm and the PDI is 0.391. The micelle was quite stable in neutral environment but fragile in acidic solution as is illustrated in Fig. 6. A certain volume of micelle solution (1.0 mg ml−1 in deionized water) was diluted by same volume of buffer solution with different pH. When diluted by the pH 7.4 buffer solution (0.1 M), the diameter of the micelle was unchanged. However, when diluted by the pH 5.0 buffer solution (0.1 M), it hydrolyzed quickly. The critical micelle concentration was studied using pyrene as the fluorescent agent. The value of the CMC is about 2.0 μg (ESI, Fig. 5†).The DOX-loaded micelles and drug release behavior
DOX-loaded HBPAs–hydrazone–PEG micelles were prepared by the dialysis method. Before drug loading, doxorubicin hydrochloride (DOX·HCl) was pre-treated with excess triethylamine in DMC overnight. The weight ratio of the DOX to micelle was 10%. Drug loading content (DLC) and drug loading efficiency (DLE) were 2.34% and 23.4%, respectively. DOX was detected by UV-VIS at predetermined wavelength of 482 nm. DLC and DLE were calculated by the following eqn (2):
 | (2) |
Compared to the blanked micelles, the particle size of drug-loaded micelles increased from 30 nm to 35 nm (Fig. 7). The increase of the diameter was mainly due to the encapsulation of the DOX.
Fig. 8 gives the drug release curve at different pH buffer solutions. DOX was released faster in pH 5.0 buffer solution than in pH 7.4 buffer solution. This is because the carrier of HBPAs–hydrazone–PEG is pH sensitive. More than 50% of DOX was released from the carrier in less than 10 hours at pH 5.0 buffer solution, whereas less than 10% of DOX was released from the carrier at pH 7.4 buffer solution. The leakage of the DOX at neutral solution is due to the hydrophilicity of HBPAs. In fact, HBPAs is different from the traditional hydrophobic materials such as PLA and PCL. There are plenty of ethenyl-oxy groups, which are hydrophilic and similar to PEG in the backbone of HBPAs, but the benzene ring bestows HBPAs with some degree of hydrophobicity. Therefore, there is a little hydrophilicity in HBPAs. Water can penetrate into the core of the micelle and swell the micelle. This is the reason why DOX could also leak out from the carrier at neutral buffer solution.
Conclusions
Amphiphilic hyperbranched polyacetals containing dual pH sensitive bonds-acetal and hydrazone bonds was synthesized by our group. Hydrolysis experiments demonstrated that it hydrolyzed quickly in acidic environment, whereas it was quite stable at neutral environment. The micelles of HBPAs–hydrazone–PEG could be easily formed by dialysis method. The micelles were stable at neutral buffer solution but hydrolyzed in acidic buffer solution. After DOX was encapsulated into the micelle, the size of the micelle increased to 35 nm from 30 nm. The drug released faster in pH 5.0 than in pH 7.4. More than 50% of DOX was released from the micelle, but there is also less than 10% of DOX leaking out from the micelle, which is mainly due to the hydrophilicity of HBPAs.Acknowledgements
This study was supported by the National Natural Science Foundation of China (21474086).Notes and references
- C. Gao and D. Yan, Prog. Polym. Sci., 2004, 29, 183 CrossRef CAS PubMed.
- A. Hult, M. Johansson and E. Malmstrom, Advances in Polymer Science: Branched polymers II, 1999, vol. 143, p. 1 Search PubMed.
- K. Inoue, Prog. Polym. Sci., 2000, 25, 453 CrossRef CAS.
- M. Jikei and M. Kakimoto, Prog. Polym. Sci., 2001, 26, 1233 CrossRef CAS.
- B. Voit, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 2505 CrossRef CAS.
- C. C. Lee, Nat. Biotechnol., 2005, 23, 1517 CrossRef CAS PubMed.
- C. Kojima, Bioconjugate Chem., 2000, 11, 910 CrossRef CAS PubMed.
- N. Malik, E. G. Evagorou and R. Duncan, Anti-Cancer Drugs, 1999, 10, 767 CrossRef CAS PubMed.
- J. F. Kukowska-Latallo, Cancer Res., 2005, 65, 5317 CrossRef CAS PubMed.
- M. J. Liu, K. Kono and J. M. J. Frechet, J. Controlled Release, 2000, 65, 121 CrossRef CAS.
- M. T. Morgan, et al., J. Am. Chem. Soc., 2003, 125, 15485 CrossRef CAS PubMed.
- H. D. King, J. Med. Chem., 2002, 45, 4336 CrossRef CAS PubMed.
- Y. H. Choe, J. Controlled Release, 2002, 79, 55 CrossRef CAS.
- G. Pasut, J. Bioact. Compat. Polym., 2005, 20, 213 CrossRef CAS PubMed.
- K. Haba, Angew. Chem., Int. Ed., 2005, 44, 716 CrossRef CAS PubMed.
- R. A. Shenoi, Biomaterials, 2013, 34, 6068 CrossRef CAS PubMed.
- S. Chatterjee and S. Ramakrishnan, Macromolecules, 2011, 44, 4658 CrossRef CAS.
- Y. Lu, Acta Polym. Sin., 2013, 3, 391 Search PubMed.
- G. C. Behera and S. Ramakrishnan, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 102 CrossRef CAS.
- Y. Lee, Bioconjugate Chem., 2008, 19, 525 CrossRef CAS PubMed.
- S. Lee, Biomacromolecules, 2012, 13, 1190 CrossRef CAS PubMed.
- D. Holter, A. Burgath and H. Frey, Acta Polym., 1997, 48, 30 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra16935b |
| This journal is © The Royal Society of Chemistry 2015 |