Self-healing and shape memory capabilities of copper-coordination polymer network†
Abstract
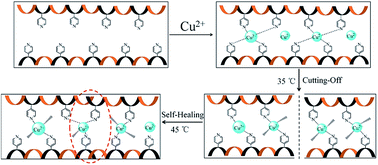
A novel smart material with excellent shape memory and high self-healing capabilities has been developed through metal ion coordinating polymer. The copper-coordination polymer network (Cu–PIE) is formed via the isonicotinate moiety of isonicotinate-functionalized polyesters (PIE) covalently cross-linking with copper ions.

 Please wait while we load your content...
Please wait while we load your content...