Sprayable, paintable layer-by-layer polyaniline nanofiber/graphene electrodes†
Abstract
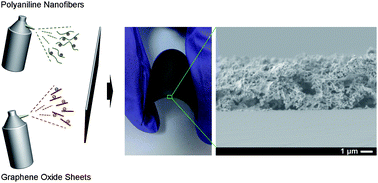
Using polyaniline nanofibers and graphene oxide sheets, we demonstrate here the successful layer-by-layer (LbL) assembly of the two anisotropic nanomaterials using a water-based spray-on approach. The processing parameters most critical to the production of uniform electrodes are blow-drying time and removal of the rinsing step. The resulting polyaniline nanofiber/graphene oxide films are electrochemically reduced to convert graphene oxide to reduced graphene oxide, as evidenced by a distinctive change in colour and Raman spectra. The architecture of the electrode is highly porous (74% void), which facilitates ion transport. The electrodes' ability to store charge is evaluated as a function of thickness in non-aqueous conditions (LiClO4 in propylene carbonate, lithium metal anode). The capacity reaches values as high as 114 mA h g−1 (45 mA h cm−3) at 0.03 A g−1. Besides capacity, other performance metrics (energy and power) are compared against control electrodes made by a different processing approach, dip-assisted LbL assembly. It is found that spray-assisted LbL assembly is over 70 times faster and yields electrodes with better rate capability relative to dip-assisted LbL assembly.
- This article is part of the themed collection: Polymers for Electrochemical Energy Storage

 Please wait while we load your content...
Please wait while we load your content...