Use of deuterium labelling—evidence of graphene hydrogenation by reduction of graphite oxide using aluminium in sodium hydroxide†
Abstract
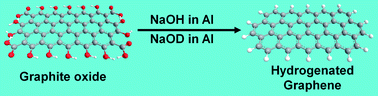
Highly hydrogenated graphene is one of the main focuses in graphene research. Hydrogenated graphene may have many unique properties such as fluorescence, ferromagnetism, and a tuneable band gap. The most widely used techniques for the fabrication of highly hydrogenated graphene are based on technically challenging methods concerning the usage of liquid ammonia reduction pathways with alkali metals or plasma hydrogenation. However, the reduction of graphene oxide by nascent hydrogen is a simple and effective method leading to the formation of highly hydrogenated graphene at room temperature. Using deuterium labelling, we studied the hydrogenation of graphene oxides prepared by chlorate and permanganate methods. The nascent hydrogen/deuterium was formed by the reaction of aluminum powder with a solution of sodium deuteroxide in deuterated water. The synthesis of hydrogenated graphene was confirmed and it was characterized in detail.

 Please wait while we load your content...
Please wait while we load your content...