Enantioselective bioreductive preparation of chiral halohydrins employing two newly identified stereocomplementary reductases†
Abstract
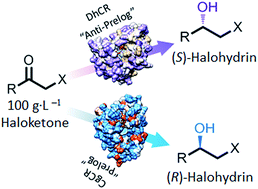
Two robust stereocomplementary carbonyl reductases (DhCR and CgCR) were identified through rescreening the carbonyl reductase toolbox. Five reductases were returned through the activity and enantioselectivity assay for α-chloro-1-acetophenone and ethyl 4-chloro-3-oxo-butanate (COBE). Three reductases were stable at elevated substrate loading. Enzymatic characterization revealed that DhCR and CgCR were more thermostable. As much as 330 g COBE in 1 L biphasic reaction mixture was reduced to (S)- and (R)-3-hydroxy-4-chlorobutyrate by DhCR and CgCR (coexpressed with glucose dehydrogenase), with 92.5% and 93.0% yields, >99% ee, and total turnover numbers of 53 800 and 108 000, respectively. Six other α-halohydrins were asymmetrically reduced to optically pure forms at a substrate loading of 100 g L−1. Our results indicate the potential of these two stereocomplementary reductases in the synthesis of valuable α-halohydrins for pharmaceuticals.

 Please wait while we load your content...
Please wait while we load your content...