Synthesis of different CuO nanostructures from Cu(OH)2 nanorods through changing drying medium for lithium-ion battery anodes†
Abstract
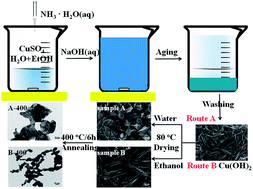
Different CuO nanostructures have been successfully synthesized through changing the drying medium of Cu(OH)2 nanorods precursors. Herein, Cu(OH)2 nanorods precursors were first prepared through a simple precipitation method. When H2O was chosen as the drying medium at 80 °C, 2D leaf-like CuO nanostructures were obtained. While dried in ethanol 1D Cu(OH)2 nanorods were obtained. With post-annealing, porous CuO nanoleaves and nanocrystalline-assembled CuO nanorods were successfully synthesized, respectively, which have been applied to lithium batteries as anode materials and influence of different nanostructures on the electrochemical properties have been investigated. Fortunately, both novel nanostructured CuO successfully displayed high capacity and excellent cycling stability, and porous CuO nanoleaves can exhibit a reversible capacity of 633 mA h g−1 and 576 mA h g−1 after 100 and 150 discharge–charge cycles at 67.4 mA g−1, which is superior to that of CuO nanoleaves without post-annealing. These results may provide valuable insights for the industrialization and development of new nanostructured anodes for next-generation high-performance lithium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...