Asphalt-assisted assembly of breath figures: a robust templating strategy for general fabrication of ordered porous polymer films
Abstract
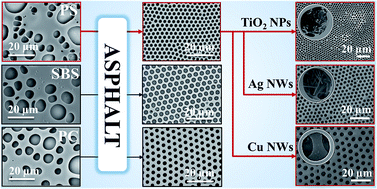
A robust asphalt-assisted breath figure templating approach has been developed for general fabrication of ordered porous polymer films and functional hybrid films with hierarchical microstructures. The incorporation of desired non-polymeric components, such as titanium dioxide nanoparticles and silver nanowires, shows little influence on the regularity of the pore arrays.

 Please wait while we load your content...
Please wait while we load your content...