Nanocrystalline ZSM-5 based bi-functional catalyst for two step and three step tandem reactions
Rajendra Srivastava*a,
Bhaskar Sarmaha and
Biswarup Satpatib
aDepartment of Chemistry, Indian Institute of Technology Ropar, Rupnagar-140001, India. E-mail: rajendra@iitrpr.ac.in; Fax: +91-1881-223395; Tel: +91-1881-242175
bSurface Physics and Material Science Division, Saha Institute of Nuclear Physics, 1/AF, Bidhannagar, Kolkata 700 064, India
First published on 5th March 2015
Abstract
A Pd nanoparticle decorated nanocrystalline ZSM-5 catalyst was prepared for one-pot tandem reactions. The catalyst was characterized by the complementary combination of X-ray diffraction, N2-adsorption, electron microscopy, and NH3-temperature programmed desorption techniques. The catalyst was investigated in the one-pot tandem conversion of benzyl alcohol to 1,3-diphenyl-3-(phenylamino)propan-1-one, (E)-chalcone, and 2,3-dihydro-1,5-benzothiazepine. 1,3-Diphenyl-3-(phenylamino)propan-1-one and (E)-chalcone were prepared by two step tandem reactions from benzyl alcohol, whereas 2,3-dihydro-1,5-benzothiazepine was prepared by a three step tandem reaction from benzyl alcohol. A recycling study shows that no significant decrease in the catalytic activity was observed even after three recycles. To the best of our knowledge, this is the first report which deals with such a simple route to prepare 2,3-dihydro-1,5-benzothiazepines in a one-pot tandem methodology from benzyl alcohol.
1. Introduction
Development of heterogeneous catalytic processes for fine and specialty chemicals is an important inter-disciplinary research objective for industrialists and academicians.1–8 Several multi-functional heterogeneous catalysts that catalyze more than one organic transformation have been reported.9–16 A unique green chemical approach for increasing the efficiency of a catalytic process is to carry out more than one catalytic transformation in a ‘one-pot’ process without isolating any of the intermediates. Such tandem or cascade reactions need to be carefully planned to ensure that the correct sequence of reactions is followed in the right order to achieve the target product.17–21 The design of a suitable multi-functional catalyst is the key for a successful tandem reaction. For the development of a multi-functional catalyst, it is important to choose a suitable support material that itself possess some catalytic activity and also has the ability to incorporate more catalytic functionalities. Our research is based on the development of ionic liquid and zeolite based catalytic process.22–28 It is possible to develop ionic liquid and zeolite based multi-functional catalysts but due to their easy recovery and efficient recycling, zeolite based bi-functional catalysts were developed for tandem reactions in this study.Zeolites play important role in catalysis, adsorption, and separation processes, because of well-defined porous crystalline structures, unique channel systems of molecular dimension, and tunable chemical compositions.29–32 Conventional microporous zeolite is not suitable for the development of multi-functional catalyst because it doesn't have large external surface area and surface silanol groups, which are important to incorporate catalytic functionality. Surface silanol groups present at large external surface of nanocrystalline/hierarchical zeolites are required for the development of multi-functional catalysts. Several pioneers in zeolite science have developed synthesis strategy to prepare hierarchical/mesoporous/nanocrystalline zeolites.33–38 Top-down and bottom-up approaches were used to prepare such zeolite materials.39 Our research group also developed several organic structure directing agents based on piperidine, imidazole, and 1,4-diazabicyclo[2.2.2]octane that acted as structure directing agents for the synthesis of zeolites of different framework structure.40–44
The objective of this study was to design and develop nanocrystalline ZSM-5 based bi-functional catalysts for two steps and three steps tandem reactions. Pd nanoparticles were supported on the surface of acidic Nano-ZSM-5 to obtain an efficient bi-functional catalyst. Catalyst was investigated in the one-pot synthesis of 1,3-diphenyl-3-(phenylamino)propan-1-one (hereafter represented as DPO), (E)-chalcone, and 2,3-dihydro-1,5-benzothiazepines (hereafter represented as DBT). To the best of our knowledge, this is the first report, which deals with the synthesis of 2,3-dihydro-1,5-benzothiazepines in one-pot tandem methodology. In conventional practice, three steps and three catalysts are needed to obtain 2,3-dihydro-1,5-benzothiazepines.
2. Experimental
2.1. Catalyst synthesis
Tetraethylorthosilicate (TEOS), sodium aluminate (53 wt% Al2O3, 43 wt% Na2O), tetrapropylammonium hydroxide (TPAOH) (1 M aqueous solution) and propyltriethoxy silane (PrTES) were used in the synthesis of zeolite. Nano-ZSM-5 with Si/Al = 20 (input) was prepared using molar composition of 90TEOS/10 PrTES/2.5 Al2O3/3.3 Na2O/25 TPAOH/2500 H2O by following the reported procedure.45,46 In a typical synthesis of nanocrystalline zeolite (Nano-ZSM-5), 1.44 g sodium aluminate (53 wt% Al2O3, 43 wt% Na2O) was dissolved in 75 mL distilled water (Solution A). 6.39 g PrTES was mixed with 75 mL of aqueous TPAOH solution (Solution B). Solution A and Solution B were mixed, and the resultant solution was stirred for 15 minutes at ambient temperature, until it became a clear solution. 57.39 g TEOS was added into the resultant solution and stirring was continued for 6 h. This mixture was transferred to a Teflon-lined autoclave, and hydrothermally treated at 443 K for 4 days under static condition. The final product was filtered, washed with distilled water, and dried at 373 K. Material was calcined at 823 K for 6 h under flowing air.5 g of obtained zeolite was then added to 200 mL, 1 M aqueous solution of NH4NO3 and stirred at 343 K for 6 h. The resultant product was filtered and washed with distilled water several times. The ion-exchange process was repeated thrice. The final product was then dried and calcined at 823 K for 6 h. For comparative study, conventional ZSM-5 with Si/Al = 20 (input) was prepared using molar composition 100TEOS/2.5 Al2O3/3.3 Na2O/25 TPAOH/2500 H2O. Bulk Si/Al ratios of the calcined Nano-ZSM-5 and ZSM-5 are given in Table 1.
| Physical properties | |||
|---|---|---|---|
| Sample | Total surface area SBET (m2 g−1) | External surface area (m2 g−1) | Total pore volume (cm3 g−1) |
| ZSM-5 | 297 | 52 | 0.21 |
| Nano-ZSM-5 | 502 | 253 | 0.46 |
| Pd (1%)/Nano-ZSM-5 | 446 | 218 | 0.38 |
| Pd (1%)/ZSM-5 | 258 | 29 | 0.18 |
| Pd (0.5%)/Nano-ZSM-5 | 478 | 240 | 0.41 |
| Pd (2%)/Nano-ZSM-5 | 437 | 206 | 0.36 |
| Chemical properties | |||||
|---|---|---|---|---|---|
| Sample | Bulk Si/Al ratio | Pd content wt% | Total acid sites NH3 desorbed (mmol g−1) | Weak acid sites (mmol g−1) | Strong acid sites (mmol g−1) |
| ZSM-5 | 20.8 | — | 0.77 | 0.39 | 0.38 |
| Nano-ZSM-5 | 21.6 | — | 0.66 | 0.38 | 0.28 |
| Pd (1%)/Nano-ZSM-5 | 22.1 | 0.94 | 0.65 | 0.38 | 0.27 |
| Pd (0.5%)/Nano-ZSM-5 | — | 0.46 | 0.66 | 0.38 | 0.28 |
| Pd (2%)/Nano-ZSM-5 | — | 1.90 | 0.63 | 0.36 | 0.27 |
| Pd (1%)/ZSM-5 | 21.0 | 0.95 | 0.75 | 0.38 | 0.37 |
0.5 g of Nano-ZSM-5 was added into 3 mL of toluene solution containing desired amount of Pd(OAc)2 (for example; for 1 wt% sample, 10.5 mg of Pd(OAc)2 was added). After stirring for 2 h at ambient temperature, the solid was centrifuged, washed with toluene, and dried in oven. Pd2+ species adsorbed on the resultant Nano-ZSM-5 was reduced with desired amount of NaBH4 (for example; for 1 wt% sample, 7 mg of NaBH4 was added) in a 10 mL solution of toluene and ethanol (v/v = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Material was centrifuged and dried in vacuum to obtain Pd nanoparticles supported on Nano-ZSM-5. In this study, three different catalysts denoted as Pd (0.5%)/Nano-ZSM-5, Pd (1%)/Nano-ZSM-5, and Pd (2%)/Nano-ZSM-5 were prepared. For comparative study, Pd (1%)/ZSM-5 was also prepared. Amount of Pd incorporated in the resultant materials was confirmed using ICP analysis, which is given in Table 1.
1). Material was centrifuged and dried in vacuum to obtain Pd nanoparticles supported on Nano-ZSM-5. In this study, three different catalysts denoted as Pd (0.5%)/Nano-ZSM-5, Pd (1%)/Nano-ZSM-5, and Pd (2%)/Nano-ZSM-5 were prepared. For comparative study, Pd (1%)/ZSM-5 was also prepared. Amount of Pd incorporated in the resultant materials was confirmed using ICP analysis, which is given in Table 1.
2.2. Catalyst characterizations
X-ray diffraction (XRD) patterns were recorded in the 2θ range of 5–60° with a scan speed of 2° min−1 on a PANalytical X'PERT PRO diffractometer using Cu Kα radiation (λ = 0.1542 nm, 40 kV, 40 mA) and a proportional counter detector. Nitrogen adsorption measurements were performed at 77 K by Quantachrome Instruments, Autosorb-IQ volumetric adsorption analyzer. Sample was out-gassed at 573 K for 3 h in the degas port of the adsorption apparatus. The specific surface area of the material was calculated from the adsorption data points obtained at P/P0 between 0.05 and 0.3 using the Brunauer–Emmett–Teller (BET) equation. The pore diameter was estimated using the non-localized density functional theory method (DFT). Scanning electron microscopy (SEM) measurements were carried out on a JEOL JSM-6610LV to investigate the morphology of the zeolites. In depth structural analysis were carried out using FEI, TF30-ST transmission electron microscope (TEM) operating at 300 kV equipped with a GATAN Orius CCD camera. The TEM is also equipped with a scanning unit and a high-angle annular dark field (HAADF) detector from Fischione (model 3000). The compositional analysis was performed using energy dispersive X-ray (EDX, EDAX Inc.) spectroscopy attachment on the TF30. The samples were dispersed in ethanol using ultrasonic bath, and dispersed sample was mounted on a carbon coated Cu grid, dried, and used for TEM measurement. For NH3-temperature programmed desorption techniques (TPD) experiments, the catalyst sample was pretreated in He (50 cm3 min−1) at 873 K for 1 h. After cooling down to 373 K, ammonia (partial pressure 100 Torr) was passed through the samples for 1 h. Then, the sample was subsequently flushed by He stream (50 cm3 min−1) at 373 K for 1 h to remove physisorbed ammonia. TPD experiments were carried out in the range of 373–1073 K at a heating rate of 10 K min−1. The ammonia concentration in the effluent was monitored by using a gold-plated, filament thermal conductivity detector.2.3. Procedure of catalytic reactions
1H NMR (CDCl3): 7.91–7.90 (m, 2H), 7.57–7.07 (m, 10H), 6.67–6.55 (m, 3H), 5.00 (dd, 1H), 4.55 (br, 1H), 3.50 (dd, 1H), 3.42 (dd, 1H).
1H NMR (CDCl3): 8.25 (d, 2H), 7.65–7.75 (m, 5H), 7.30–7.55 (m, 7H), 5.20 (dd, 1H), 3.50 (dd, 1H), 3.25 (t, 1H).
The one-pot tandem oxidation–condensation reaction for the synthesis of (E)-chalcone was carried out in a glass flask equipped with a condenser. In a typical run, benzyl alcohol (1 mmol), catalyst (100 mg), n-dodecane (0.5 mmol, internal standard), and toluene (5 mL) were heated at 358 K for 9 h, during which oxygen was charged into the reaction mixture at flow rate of 10 mL min−1. After the reaction, the oxygen flow was stopped and toluene was removed by distillation. Acetophenone (0.12 g, 1.0 mmol) was charged into the mixture rapidly. Reaction mixture was further stirred for 7 h at 373 K. After the reaction, solid catalyst was removed by centrifugation, the reaction products were analyzed by GC and GC-MS.
3. Results and discussion
3.1. Physico-chemical characterization
Nano-ZSM-5 and ZSM-5 exhibited MFI framework structure with high phase purity, which was confirmed by XRD (Fig. 1a). XRD pattern of Nano-ZSM-5 was broad when compared to ZSM-5, which confirms that the material is nanocrystalline in nature. XRD pattern of Pd (1%)/Nano-ZSM-5 was found to be similar to Nano-ZSM-5 (Fig. 1b). No separate phase corresponding to Pd nanoparticles was observed in the XRD pattern, which confirms that Pd nanoparticles are uniformly dispersed in Nano-ZSM-5 matrix. Textural properties of the material were investigated by nitrogen adsorption–desorption measurements (Fig. 1b). Nano-ZSM-5 and Pd (1%)/Nano-ZSM-5 exhibited type-IV isotherms similar to that of mesoporous materials (Fig. 1b). The major difference in the isotherm of Nano-ZSM-5/Pd (1%)/Nano-ZSM-5 when compared to ZSM-5 is a distinct increase of N2-adsorption in the region 0.4 < P/P0 < 0.95, which is interpreted as a capillary condensation in the inter-crystalline mesopore void spaces. Bi-modal pore size distribution was obtained for Nano-ZSM-5 samples using non-localized DFT method, which confirms the presence of ZSM-5 micropores and inter-crystalline mesopores. Mesopores show a pore size distribution in the range of 2–10 nm in these materials. Textural properties obtained from N2-adsorption study are summarized in Table 1. | ||
| Fig. 1 (a) XRD patterns of various catalysts investigated in this study. (b) N2-adsorption isotherms of various catalysts investigated in this study. Inset shows pore size distribution. | ||
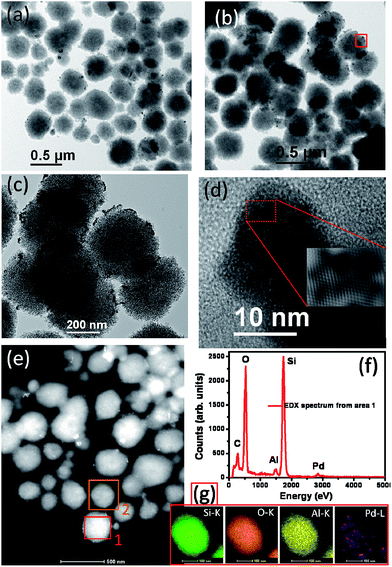
Uniform spherical particles were observed for Nano-ZSM-5 (Fig. 2a). Morphology of Pd (1%)/Nano-ZSM-5 was found to be similar to Nano-ZSM-5 (Fig. 2b). To obtain the in-depth information about nano-structure of bi-functional catalyst Pd (1%)/Nano-ZSM-5, TEM investigations were made. TEM images clearly show that Pd nanoparticles are uniformly dispersed on the surface of Nano-ZSM-5 (Fig. 3a–c). Size of Pd nanoparticles was in the range of 5–10 nm as shown in the magnified TEM image in Fig. 3d from area shown in Fig. 3b. TEM images also confirmed that the large spherical Nano-ZSM-5 particles were built with small zeolite nanocrystals of 15–20 nm (Fig. 3c). To investigate the chemical composition of the dots and surrounding matrix, Scanning TEM (STEM)-HAADF-EDX analysis was performed (Fig. 3e–g). In the HAADF image (Fig. 3e), the Z-contrast image shows Pd particles brighter than Nano-ZSM-5 particles. The elemental presence of Si, O, Al, and Pd are shown in EDX spectrum in Fig. 3f, which is from the rectangular area 1 marked in Fig. 3e. For a detailed distribution of atomic contents in the materials, elemental mapping was performed using STEM-HAADF-EDX technique (Fig. 3g). Based on the TEM imaging and elemental mapping, it can be concluded that Pd nanoparticles decorated Nano-ZSM-5 material was successfully prepared.
Acidity of the sample was investigated using NH3-TPD (Fig. 4). The total acidity decreases in the order: ZSM-5 ≈ Pd (1%)/ZSM-5 > Nano-ZSM-5 ≈ Pd (1%)/Nano-ZSM-5 (Table 1). In the TPD profiles of ZSM-5 and Nano-ZSM-5, two well-resolved symmetric peaks were observed. They dominate with maximum at 460 K & 630 K for ZSM-5 and 455 K & 650 K for Nano-ZSM-5. These peaks demonstrate the presence of weak acid sites and strong acid sites. In Nano-ZSM-5, the first peak becomes smaller and the maxima of second peak shifts to slightly higher temperature (650 K). This indicates that the acid sites in Nano-ZSM-5 are stronger than the acid sites present in ZSM-5.
During the synthesis of Nano-ZSM-5, additive propyltriethoxysilane disfavoured the extended growth of large zeolite crystal, which resulted in the formation of very small size Nano-ZSM-5. Mesopores were created by the crystal packing of these zeolite nanocrystals. Mesopore size distribution obtained from N2-adsorption studies confirmed that they were in the range of 2–10 nm. Based on SEM, TEM and N2-adsorption studies, one can conclude that Pd nanoparticles decorated spherical Nano-ZSM-5 was prepared, and the spherical Nano-ZSM-5 is composed of several zeolite nanoparticles of approximately 15–20 nm.
3.2. Catalytic investigation
The aim of this study was to develop zeolite based catalysts in the one-pot tandem conversion of benzyl alcohol to DPO, (E)-chalcone, and DBT (Scheme 1). DPO and (E)-chalcone can be prepared in two steps synthesis protocol from benzyl alcohol, whereas synthesis of DBT requires three steps from benzyl alcohol. The first step of these reactions is the oxidation of benzyl alcohol to benzaldehyde. The first step requires an oxidation catalyst. Pd nanoparticles are known to catalyze the oxidation of benzyl alcohol.47–49 Therefore, Pd nanoparticles supported Nano-ZSM-5 and ZSM-5 based catalysts were prepared and investigated in the oxidation of benzyl alcohol in the first step of the reaction (Table 2). In all the cases, benzaldehyde was obtained as selective product. Amount of catalyst, reaction temperature, and reaction atmosphere play important role in the oxidation of benzyl alcohol. Reaction proceeds well in O2 atmosphere using 100 mg of catalyst per mmol of substrate in 9 h at 358 K. After the reaction, catalyst was removed and the reaction mixture was subjected to ICP analysis. Pd was not detected in the ICP analysis. Pd (1%)/Nano-ZSM-5 was reused several times in the oxidation reaction of benzyl alcohol. After each experiment, catalyst was washed with toluene and dried in oven and then used in the next cycle. Activity of Pd (1%)/Nano-ZSM-5 was remained the same even after three recycles (Table 2). Heterogeneity and Pd-leaching of this catalyst was also examined by the ‘hot filtration’ test. Catalyst was removed from the reaction mixture after 4 h (49% benzyl alcohol conversion) then the reaction was continued for an additional 5 h gave a final benzyl alcohol conversion of 49.6%. This experiment further confirmed that the Pd was not leached during the reaction. Activity of Pd (1%)/Nano-ZSM-5 was found to be more when compared to Pd (1%)/ZSM-5. These results show that highly dispersed Pd nanoparticles were formed on the high surface area Nano-ZSM-5, which is responsible for the high catalytic activity of Pd (1%)/Nano-ZSM-5. Furthermore, enhanced diffusion provided by inter-crystalline mesopores of Nano-ZSM-5 is also responsible for the high activity of Pd (1%)/Nano-ZSM-5. | ||
| Scheme 1 One-pot tandem conversion of benzyl alcohol to 1,3-diphenyl-3-(phenylamino)propan-1-one, (E)-chalcone, and 2,3-dihydro-1,5-benzothiazepine. | ||
| S. no. | Catalyst | Catalyst amount | Atmosphere | Temp. (K) | Yielda (%) | Average TONc |
|---|---|---|---|---|---|---|
| a Yield = conversion obtained from GC (because benzaldehyde was obtained as only product).b Data obtained after third recycle.c Average TON = turnover number [moles of benzyl alcohol converted per mole of active Pd].d Reaction condition: benzyl alcohol (1 mmol), time (9 h), O2 flow (10 mL min−1), toluene (5 mL), n-dodecane (0.5 mmol, internal standard). | ||||||
| 1 | Pd (1%)/Nano-ZSM-5 | 100 mg | O2 | 358 | 80 | 91 |
| 2 | Pd (1%)/Nano-ZSM-5 | 150 mg | O2 | 358 | 98 | 74 |
| 3 | Pd (1%)/Nano-ZSM-5 | 50 mg | O2 | 358 | 49 | 111 |
| 4 | Pd (1%)/Nano-ZSM-5 | 100 mg | Air | 383 | 43 | 49 |
| 5 | Pd (1%)/Nano-ZSM-5 | 100 mg | O2 | 313 | 8 | 9 |
| 6 | Pd (1%)/Nano-ZSM-5 | 100 mg | O2 | 333 | 46 | 52 |
| 7 | Pd (1%)/Nano-ZSM-5 | 100 mg | O2 | 383 | 96 | 109 |
| 8 | Pd (0.5%)/Nano-ZSM-5 | 100 mg | O2 | 358 | 44 | 102 |
| 9 | Pd (2%)/Nano-ZSM-5 | 100 mg | O2 | 358 | 96 | 57 |
| 10 | Pd (1%)/Nano-ZSM-5b | 100 mg | O2 | 358 | 81 | 92 |
| 11 | Pd (1%)/ZSM-5 | 100 mg | O2 | 358 | 55 | 63 |
Before the investigation of one-pot tandem reaction starting with benzyl alcohol using bi-functional catalysts, it would be interesting to obtain catalytic data using ZSM-5, Nano-ZSM-5, Pd (1%)/Nano-ZSM-5, and Pd (1%)/ZSM-5 for the production of DPO, (E)-chalcone, and DBT from benzaldehyde. Role of these catalysts was investigated in the synthesis of DPO by following the Mannich condensation of aniline with benzaldehyde and acetophenone (Table 3). Significantly low product yield was obtained in the absence of catalyst under the optimized reaction condition. When ZSM-5 was used as catalyst, only slight increase in the DPO yield was observed. Nano-ZSM-5 produced very good DPO yield under mild reaction condition. Pd (1%)/Nano-ZSM-5 produced similar DPO yield when compared to Nano-ZSM-5. This clearly shows that Pd has no role to play in the Mannich reaction. The role of these catalysts was investigated in the synthesis of (E)-chalcone by the aldol condensation of benzaldehyde and acetophenone (Table 3). In the absence of catalyst, no reaction took place under the optimized reaction condition. ZSM-5 exhibited significantly low activity when compared to Nano-ZSM-5. Activity of Nano-ZSM-5 in the synthesis of (E)-chalcone was low when compared to DPO. The activity of Pd (1%)/Nano-ZSM-5 was found to be similar to Nano-ZSM-5. Activity of Nano-ZSM-5 in the synthesis of (E)-chalcone can be improved by performing the reaction at 373 K or using more amount of catalyst (Table 3, compare entry no. 6–8 with entry 3). Furthermore, when (E)-chalcone was reacted with 2-aminothiophenol, it formed DBT in the presence of various catalysts investigated in this study. Reaction proceeded even in the absence of catalyst. However, product yield was very low. Product yield was improved when ZSM-5 was used as catalyst. The activity of Nano-ZSM-5 was found to be exceptionally high in the synthesis of DBT when compared to DPO and (E)-chalcone (Table 3). The activity of Pd (1%)/Nano-ZSM-5 was found to be similar to that of Nano-ZSM-5 (Table 3). To the best of our knowledge, we could find only one report in which wide range of acid catalysts were reported for the synthesis of DBT.50 The activity of Nano-ZSM-5 was found to be comparable or better when compared to these catalysts reported in the literature.50 Recycling study confirmed that Nano-ZSM-5 was found to be reusable and exhibited almost similar activity even after three recycles (Table 3). Activity of Nano-ZSM-5 was found to be more than ZSM-5 in the acid catalyzed condensation reactions described above. This difference in the activity can be correlated to high surface area and inter-crystalline mesopores. In the case of Nano-ZSM-5, the acid sites located at the pore-mouth or little bit farther inside the channels (which may be called subsurface centers) are the real centers of the reaction. Inter-crystalline mesopores present in the Nano-ZSM-5 facilitate the diffusion of reactant to the acid sites and diffusion of the product from the active sites, which is responsible for the high activity.
| Synthesis of DPO | ||
|---|---|---|
| S. no. | Catalyst | DPO yielda (%) |
| a Yield = isolated yield.b Yield = conversion obtained from GC (E-chalcone was obtained as only product).c Catalyst (100 mg).d Temperature (373 K).e Catalyst (100 mg), temperature (373 K).f Data obtained after third recycle.g Reaction condition: synthesis of DPO: benzaldehyde (4 mmol), acetophenone (4 mmol), aniline (4 mmol), catalyst (50 mg), toluene (5 mL), temperature (303 K), time (7 h). Synthesis of (E)-chalcone: benzaldehyde (1.5 mmol), acetophenone (1.5 mmol), catalyst (50 mg), temperature (323 K), time (7 h). Synthesis of DBT: (E)-chalcone (1 mmol), 2-aminothiophenol (1.2 mmol), catalyst (50 mg), toluene (8 mL), temperature (383 K), time (2 h). | ||
| 1 | None | 5.7 |
| 2 | ZSM-5 | 13.4 |
| 3 | Nano-ZSM-5 | 88 |
| 4 | Pd (1%)/Nano-ZSM-5 | 82 |
| 5 | Pd (1%)/ZSM-5 | 12.6 |
| 6 | Nano-ZSM-5f | 85.7 |
The catalytic activity of Pd (1%)/Nano-ZSM-5 and Pd (1%)/ZSM-5 in the one-pot tandem synthesis of DPO/(E)-chalcone from benzyl alcohol was studied. The results are listed in Table 4. The one-pot synthesis of DPO was realized through the oxidation of benzyl alcohol followed by Mannich condensation with aniline and acetophenone. The one-pot synthesis of (E)-chalcone was realized through the oxidation of benzyl alcohol followed by aldol condensation with acetophenone. Benzaldehyde was produced as an intermediate and DPO/(E)-chalcone was the final product. Reaction did not take place in the absence of catalyst. Furthermore, DPO/(E)-chalcone was not formed using Nano-ZSM-5 catalyst in one-pot tandem synthesis from benzyl alcohol. In one-pot tandem synthesis of DPO/(E)-chalcone, Pd (1%)/Nano-ZSM-5 was found to exhibit very good catalytic activity. Excellent benzyl alcohol conversion and very good selectivity for DPO/(E)-chalcone was achieved using Pd (1%)/Nano-ZSM-5 when compared to Pd (1%)/ZSM-5.
| Oxidation–Mannich condensation | |||||
|---|---|---|---|---|---|
| S. no. | Catalyst | Time (T1 + T2) | Benzyl alcohol conv. (%) | Product yield (%) | |
| Benzaldehyde | DPO | ||||
| 1 | Nil | 9 + 7 | 0 | — | — |
| 2 | Pd (1%)/ZSM-5 | 9 + 7 | 54.6 | 35.7 | 18.9 |
| 3 | Pd (1%)/Nano-ZSM-5 | 9 + 7 | 81.8 | 6.7 | 75.1 |
| Oxidation–aldol condensation | |||||
|---|---|---|---|---|---|
| S. no. | Catalyst | Time (T1 + T2) | Benzyl alcohol conv. (%) | Product yield (%) | |
| Benzaldehyde | E-Chalcone | ||||
| 1 | Nil | 9 + 7 | 0 | — | — |
| 2 | Pd (1%)/ZSM-5 | 9 + 7 | 55.3 | 45.9 | 9.4 |
| 3 | Pd (1%)/Nano-ZSM-5 | 9 + 7 | 81.4 | 7.8 | 73.6 |
Finally, one-pot synthesis of DBT was realized through the oxidation of benzyl alcohol followed by aldol condensation with acetophenone and the reaction of (E)-chalcone with 2-aminothiophenol (Table 5). Benzaldehyde was produced as first intermediate and (E)-chalcone was the second intermediate. At the end of the reaction, four products were obtained in the GC analysis. They were remaining amounts of benzaldehyde and (E)-chalcone. Apart from the desired product DBT, one additional product 2-phenylbenzothiazole was also obtained. 2-Phenylbenzothiazole was formed by the reaction of benzaldehyde and 2-aminothiophenol. No reaction took place in the absence of catalyst. In contrast to the synthesis of DBT from benzaldehyde, in this one-pot tandem synthesis from benzyl alcohol, DBT was not formed in the presence of Nano-ZSM-5. In one-pot tandem synthesis of DBT, Pd (1%)/Nano-ZSM-5 exhibited excellent benzyl alcohol conversion and good DBT selectivity.
| S. no. | Catalyst | Time (T1 + T2 + T3) (h) | Benzyl alcohol conv. (%) | Product yield (%) | |||
|---|---|---|---|---|---|---|---|
| Benzaldehyde | (E)-Chalcone | DBT | Othera | ||||
| a 2-Phenylbenzothiazole.b Data obtained after third recycle. | |||||||
| 1 | Nil | 9 + 7 + 2 | 0 | — | — | — | — |
| 2 | Pd (1%)/Nano-ZSM-5 | 9 + 7 + 2 | 80.6 | 0.8 | 1.4 | 67.0 | 11.4 |
| 3 | Pd (1%)/ZSM-5 | 9 + 7 + 2 | 55.1 | 16.8 | 5.4 | 4.3 | 28.6 |
| 4 | Pd (1%)/Nano-ZSM-5b | 9 + 7 + 2 | 81.2 | 1.0 | 1.9 | 67.4 | 10.9 |
Pd/ZSM-5 is widely investigated in organic synthesis, for example oxidation of volatile organic compounds, oxidation of benzyl alcohol, coupling reactions etc.51,52 In this study Pd/Nano-ZSM-5 was investigated for the one-pot tandem reactions. Recently, an effort was made to prepare Pd nanoparticles supported organic–inorganic hybrid ZSM-5 based bi-functional catalysts, which catalyzed one-pot synthesis of benzylidene malononitrile from benzyl alcohol under soluble-base-free conditions, in which the benzyl alcohol was first oxidized to intermediate benzaldehyde followed by Knoevenagel condensation with malononitrile.53 Activity of catalysts investigated in this study for the oxidation of benzyl alcohol under O2 atmosphere was comparable with the catalyst reported in the literature.53 It may be noted that Pd supported SiO2 exhibited very low activity in the oxidation of benzyl alcohol.54 However, the catalytic activity was improved by incorporating FeOx.54 Literature reports suggest that bi-metallic catalysts such as Pd–Au is good for the oxidation of benzyl alcohol under O2 atmosphere.55 In this study, we provided a simple and straight forward route for the synthesis Pd nanoparticles supported Nano-ZSM-5, which exhibited very good activity in the oxidation of benzyl alcohol. Furthermore, Pd/Nano-ZSM-5 is reported here for the first time in the one-pot two steps synthesis of 1,3-diphenyl-3-(phenylamino)propan-1-one and (E)-chalcone from benzyl alcohol or one-pot three steps synthesis of 2,3-dihydro-1,5-benzothiazepine from benzyl alcohol. Excellent activity of Pd/Nano-ZSM-5 in one-pot tandem reaction can be correlated to highly dispersed Pd nanoparticles on high surface area Nano-ZSM-5, inter-crystalline mesopores and strong acid sites located at pore mouth or little bit further inside the porous channels of Nano-ZSM-5.
4. Conclusions
2,3-Dihydro-1,5-benzothiazepines was prepared in one-step from (E)-chalcone in excellent yield using Nano-ZSM-5 catalyst. Furthermore, 1,3-diphenyl-3-(phenylamino)propan-1-one and (E)-chalcone were also prepared from benzaldehyde in one-step using Nano-ZSM-5 in good yields. These products were successfully prepared in one-pot tandem reaction protocol from benzyl alcohol. Pd nanoparticles supported on Nano-ZSM-5 based bi-functional catalyst was successfully prepared for one-pot, two steps/three steps tandem reactions to obtain these products. Pd nanoparticles supported on Nano-ZSM-5 was found to be an efficient catalyst in these tandem reactions. High surface area and surface silanol groups were favorable for the dispersion of Pd nanoparticles on its surface to produce highly efficient bi-functional catalysts. Furthermore, inter-crystalline mesopores present in the Nano-ZSM-5 facilitate the diffusion of reactant to the acid sites and diffusion of the product from the active sites, which is responsible for the high activity of Pd nanoparticles supported on Nano-ZSM-5 in the synthesis of these chemicals. ICP analysis and recycling study confirmed that no significant loss in the catalytic active Pd species and catalytic activity were observed even after three recycles.Acknowledgements
Authors thank Department of Science and Technology, New Delhi for financial assistance (DST grant SB/S1/PC-91/2012). BS is grateful to UGC, New Delhi for JRF fellowship.References
- F. Su and Y. Guo, Green Chem., 2014, 16, 2934–2957 RSC
.
- B. S. Kumar, A. Dhakshinamoorthy and K. Pitchumani, Catal. Sci. Technol., 2014, 4, 2378–2396 CAS
.
- J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C. Y. Su, Chem. Soc. Rev., 2014, 43, 6011–6061 RSC
.
- A. Dhakshinamoorthy and H. Garcia, Chem. Soc. Rev., 2014, 43, 5750–5765 RSC
.
- B. Xin and J. Hao, Chem. Soc. Rev., 2014, 43, 7171–7187 RSC
.
- Y. Zhou, G. Chen, Z. Long and J. Wang, RSC Adv., 2014, 4, 42092–42113 RSC
.
- G. Fan, F. Li, D. G. Evans and X. Duan, Chem. Soc. Rev., 2014, 43, 7040–7066 RSC
.
- F. Lee, J. A. Bennet, J. C. Manayil and K. Wilson, Chem. Soc. Rev., 2014, 43, 7887–7916 RSC
.
- B. Voit, Angew. Chem., Int. Ed., 2006, 45, 4238 CrossRef CAS PubMed
.
- S. Shylesh, A. Wagner, A. Seifert, S. Ernst and W. R. Thiel, Chem.–Eur. J., 2009, 15, 7052 CrossRef CAS PubMed
.
- U. Díaz and A. Corma, Dalton Trans., 2014, 10292–10316 RSC
.
- K. Motokura, M. Tada and Y. Iwasawa, J. Am. Chem. Soc., 2007, 129, 9540 CrossRef CAS PubMed
.
- A. El. Kadib, K. Molvinger, M. Bousmina and D. Brunel, J. Catal., 2010, 273, 147 CrossRef PubMed
.
- R. Srivastava, D. Srinivas and P. Ratnasamy, J. Catal., 2005, 233, 1–15 CrossRef CAS PubMed
.
- N. R. Shiju, A. H. Alberts, S. Khalid, D. R. Brown and G. Rothenberg, Angew. Chem., Int. Ed., 2011, 50, 9615–9619 CrossRef CAS PubMed
.
- Y. Yang, X. Liu, X. Li, J. Zhao, S. Bai, J. Liu and Q. Yang, Angew. Chem., Int. Ed., 2012, 51, 9164 CrossRef CAS PubMed
.
- F. X. Felpin and E. Fouquet, ChemSusChem, 2008, 1, 718 CrossRef CAS PubMed
.
- M. J. Climent, A. Corma and S. Iborra, ChemSusChem, 2009, 2, 500 CrossRef CAS PubMed
.
- M. J. Climent, A. Corma and S. Iborra, Chem. Rev., 2011, 111, 1072 CrossRef CAS PubMed
.
- M. J. Climent, A. Corma, S. Iboarra and M. J. Sabater, ACS Catal., 2014, 4, 870–891 CrossRef CAS
.
- J. M. Fraile, N. García, C. I. Herrerías and J. A. Mayoral, Catal. Sci. Technol., 2013, 3, 436–443 CAS
.
- R. Kore and R. Srivastava, Catal. Commun., 2011, 12, 1420–1424 CrossRef CAS PubMed
.
- R. Kore and R. Srivastava, J. Mol. Catal. A: Chem., 2011, 345, 117–126 CrossRef CAS PubMed
.
- R. Kore, T. J. D. Kumar and R. Srivastava, J. Mol. Catal. A: Chem., 2012, 360, 61–70 CrossRef CAS PubMed
.
- R. Kore and R. Srivastava, J. Mol. Catal. A: Chem., 2013, 376, 90–97 CrossRef CAS PubMed
.
- R. Kore, M. Tumma and R. Srivastava, Catal. Today, 2012, 198, 189–196 CrossRef CAS PubMed
.
- R. Kore, R. Srivastava and B. Satpati, ACS
Catal., 2013, 12, 2891–2904 Search PubMed
.
- R. Kore, B. Satpati and R. Srivastava, Appl. Catal., A, 2014, 477, 8–17 CrossRef CAS PubMed
.
- A. Corma, Chem. Rev., 1997, 97, 2373–2420 CrossRef CAS PubMed
.
- C. S. Cundy and P. A. Cox, Chem. Rev., 2003, 103, 663–702 CrossRef CAS PubMed
.
- A. Primo and H. Garcia, Chem. Soc. Rev., 2014, 43, 7548–7561 RSC
.
- W. J. Roth, P. Nachtigall, R. E. Morris and J. Čejka, Chem. Rev., 2014, 114, 4807–4837 CrossRef CAS PubMed
.
- Y. Tao, H. Kanoh, L. Abrams and K. Kaneko, Chem. Rev., 2006, 106, 896–910 CrossRef CAS PubMed
.
- R. Chal, C. Gérardin, M. Bulut and S. vanDonk, ChemCatChem, 2011, 3, 67–81 CrossRef CAS
.
- J. P. Ramirez, C. H. Christensen, K. Egeblad, C. H. Christensen and J. C. Groen, Chem. Soc. Rev., 2008, 37, 2530–2542 RSC
.
- L. H. Chen, X. Y. Li, J. C. Rooke, Y.-H. Zhang, X. Y. Yang, Y. Tang, F. S. Xiao and B. L. Su, J. Mater. Chem., 2012, 22, 17381–17403 RSC
.
- K. Na, M. Choi and R. Ryoo, Microporous Mesoporous Mater., 2013, 166, 3–19 CrossRef CAS PubMed
.
- K. Li, J. Valla and J. Garcia-Martinez, ChemCatChem, 2014, 6, 46–66 CrossRef CAS
.
- K. Moller and T. Bein, Chem. Soc. Rev., 2013, 42, 3689–3707 RSC
.
- R. Kore, B. Satpati and R. Srivastava, Chem.–Eur. J., 2011, 17, 14360–14365 CrossRef CAS PubMed
.
- R. Kore and R. Srivastava, RSC Adv., 2012, 2, 10072–10084 RSC
.
- R. Kore and R. Srivastava, Catal. Commun., 2012, 18, 11–15 CrossRef CAS PubMed
.
- R. Kore, R. Sridharkrishna and R. Srivastava, RSC Adv., 2013, 3, 1317–1322 RSC
.
- R. Kore, R. Srivastava and B. Satpati, Chem.–Eur. J., 2014, 36, 11511–11521 CrossRef PubMed
.
- R. Srivastava, N. Iwasa, S. I. Fujita and M. Arai, Chem.–Eur. J., 2008, 14, 9507–9511 CrossRef CAS PubMed
.
- B. Kaur, M. U. A. Prathap and R. Srivastava, ChemPlusChem, 2012, 77, 1119–1127 CrossRef CAS
.
- K. Hara, S. Tayama, H. Kano, T. Masuda, S. Takakusagi, T. Kondo, K. Uosaki and M. Sawamura, Chem. Commun., 2007, 4280–4282 RSC
.
- S. E. Davis, M. S. Ide and R. J. Davis, Green Chem., 2013, 15, 17–45 RSC
.
- H. Wang, S. X. Deng, Z. R. Shen, J. G. Wang, D. T. Ding and T. H. Chen, Green Chem., 2009, 11, 1499–1502 RSC
.
- M. J. Climent, A. Corma, S. Iborra and L. Marti, ChemSusChem, 2014, 7, 1177–1185 CrossRef CAS PubMed
.
- C. He, J. Le, J. Cheng, L. Li, P. Li, Z. Hao and Z. P. Xu, Ind. Eng. Chem. Res., 2009, 48, 6930–6936 CrossRef CAS
.
- M. Opanasenko, P. Štěpnička and J. Čejka, RSC Adv., 2014, 4, 65137–65162 RSC
.
- L. Xu, C.-G. Li, K. Zhang and P. Wu, ACS Catal., 2014, 4, 2959–2968 CrossRef CAS
.
- L. Li, J. Zhao, J. Yang, T. Fu, N. Xue, L. Peng, X. Guo and W. Ding, RSC Adv., 2015, 5, 4766–4769 RSC
.
- X. Yang, C. Huang, Z. Fu, H. Song, S. Liao, Y. Su, L. Du and X. Li, Appl. Catal., B, 2013, 140, 419–425 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |