Superantigenicity analysis of staphylococcal enterotoxins SElK and SElQ in a mouse model
Abstract
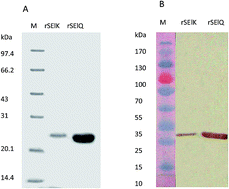
Staphylococcal enterotoxins (SEs) are superantigenic toxins secreted by Staphylococcus aureus that is involved in causing food poisoning and human diseases. So far, more than 20 genotypes of SE and SE-like proteins (SEls) have been identified. While many SEs have been found to be capable of causing food poisoning, the role of SEIs in food and human health is still largely unknown. In this study, we analyzed the superantigenic activity of two new types of recombinant SEIs, SElK and SElQ, in a mouse model. The results show that the rSEIK and rSEIQ stimulated distinct murine T-lymphocyte proliferation, caused tumefaction in mouse spleen and thymus, SEls induced increase of cytokines (IL-2, IL-4, IL-6, TNF-α, IFN-γ) measured by quantitative PCR and ELISA both in vitro and in vivo. The results showed that the rSEIQ displayed stronger superantigenicity than the rSEIK and all caused cytokine storm and inflammatory syndromes. The molecular basis for the difference in the superantigenicity was further analyzed by 3D structural modeling. The structural difference (e.g. the critical amino acids of the α3–β8 loop) might partially explain the distinct immune-stimulatory activity of rSElK and rSElQ.

 Please wait while we load your content...
Please wait while we load your content...