In situ synthesis of amorphous RuO2/AZO as a carbon-free cathode material for Li–O2 batteries†
Mihye Wu*,
Ju Young Jo,
Sungho Choi,
Yongku Kang and
Ha-Kyun Jung*
Advanced Materials Division, Korea Research Institute of Chemical Technology, 141 Gajeongro, Yuseong, Daejeon 305-343, Korea. E-mail: wumihye@krict.re.kr; hakyun@krict.re.kr
First published on 27th February 2015
Abstract
The composite of amorphous RuO2 as an electrocatalyst and aluminum-doped ZnO (AZO) as a cathode material was synthesised and developed into a carbon-free cathode material for Li–O2 batteries for the first time. The amorphous RuO2/AZO carbon-free cathode exhibits a noticeably reduced overpotential as well as an enhanced specific capacity.
Despite the enormous energy density of Li–O2 batteries, which is 3–5 times greater than that of Li-ion batteries,1 they are associated with known limitations, such as degradation of the electrolyte, poor cycle stability, and a low round-trip efficiency, among others.2 Among these shortcomings, the formation of lithium carbonate (Li2CO3) is one of the main problems leading to the deterioration of the electrochemical properties of Li–O2 batteries. Because carbon is the most commonly used cathode material, lithium carbonates are produced and deposited onto the surface of the cathode during discharge process and do not easily decompose during the charge process. As a result, the available pores on the cathode are blocked, leading to capacity fading during cycling. The formation of lithium carbonates is based on the carbon cathode and on electrolytes to form a Li2CO3 layer at the interface between the carbon cathode and the Li2O2.3 In addition, Li2CO3 decomposes into CO2 gas at the end of charge process.4 Consequently, preventing the formation of lithium carbonate must be done to enhance the electrochemical properties, which is why conductive oxides are considered as a cathode material. Li et al. reported that Ru/ITO can be applied as a carbon-free cathode material for Li–O2 batteries owing to its excellent electrochemical properties, such as its much lower charging overpotential and better cyclability.5 In this study, we employed RuO2 as an electrocatalyst and aluminum-doped ZnO (AZO) as a cathode material. AZO is chemically and physically stable and is much less expensive than indium-based conductive oxide. The electrical conductivity of AZO was increased by doping Al3+ onto Zn2+ sites, contributing to the number of electrons involved in the electron formation process by means of the interstitial zinc atoms and the oxygen vacancies.6 However, despite the fact that these materials show electrochemical activity toward Li–O2 batteries, there are additional steps needed to introduce an electrocatalyst onto cathode materials in this case. Therefore, to reduce the number of synthesis steps and to enhance the electrochemical performance levels of the carbon-free cathode material, we developed an in situ microwave-assisted hydrothermal method, which has benefits such as a simple and facile reaction process, a low reaction temperature, and the realization of uniformity in the products, as the reaction occurs at the molecular level.7 In addition, the contact between the electrocatalyst RuO2 and the cathode material AZO can be enhanced by the in situ synthesis approach due to the direct reaction among the starting materials. In preparing the RuO2/AZO, the crystallinity was controlled by varying the annealing temperature, after which the corresponding electrochemical performances were examined.
RuO2/AZO was synthesized with a microwave-assisted hydrothermal method by reacting aluminum nitrate nonahydrate, zinc nitrate hexahydrate and ruthenium chloride hydrate (for detailed synthesis method, please see the (ESI†)). The precursor powder was annealed at temperatures of 350, 400, and 500 °C in an oxidative atmosphere to obtain the RuO2/AZO powder.
The crystal structures of the synthesized RuO2/AZO particles were studied. Fig. 1 shows the XRD patterns of RuO2/AZO corresponding to the annealing temperature, which ranged from 350 °C to 500 °C in this study. All samples annealed at a given temperature include diffraction patterns representing zinc oxide (JCPDS#01-070-2551). However, the annealing temperature affected the diffraction patterns of the RuO2. The RuO2/AZO particles annealed at 400 and 500 °C include diffraction patterns corresponding to RuO2 (JCPDS#01-071-4825), showing the formation of a crystalline phase, whereas those annealed at 350 °C show no diffraction patterns except for zinc oxide, thus expressing the amorphous nature of RuO2. Therefore, RuO2/AZO annealed at 350 °C is a composite of amorphous RuO2 and crystalline AZO, while others are composites of both crystalline RuO2 and AZO.
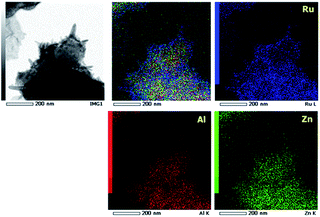
The microstructural characteristics of the RuO2/AZO prepared at different temperatures were observed by transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDS) mapping. As shown in the TEM images (Fig. S1 in ESI†), all of the particles of RuO2/AZO prepared at different temperature are composed of nanorods with a diameter of about 20 nm and nanoparticles with diameters of several nanometers. The EDS mapping was carried out on these particles to clarify the distribution of each element (Fig. 2). The signals of Al, Zn and Ru, represented as bright colored spots, were noted, and all of the elements were found to be well distributed within the particles. However, the mapping images taken from both nanorods and nanoparticles indicate the different contribution of the elements. In the nanorod-region, only blue spots, which correspond with the Ru element, were observed, whereas the red and green spots correspond with Al and Zn were not detected. In addition, the mainly distributed elements in the nanoparticle-region were Al and Zn. Considering the XRD results in Fig. 1, it can be concluded that the nanoparticles and nanorods correspond to AZO and RuO2, respectively, and both materials are evenly mixed. Therefore, unlike the RuO2/AZO prepared at temperatures greater than or equal to 400 °C, resulting in a microstructure of both crystalline RuO2 and AZO, the RuO2/AZO prepared at 350 °C is composed of amorphous RuO2 and crystalline AZO.
Because the electrochemical properties of Li–O batteries strongly depend on the surface properties, such as the surface area and pore volume, the Brunauer–Emmett–Teller (BET) technique was employed (Table S1 in ESI†). According to the results, the surface area and pore volume of RuO2/AZO gradually increased as the annealing temperature was increased. The amorphous RuO2/AZO has a surface area of 17.1 mg2 g−1 and a pore volume of 0.046 cm3 g−1, whereas all of the crystalline RuO2/AZO samples have values which are higher than this, indicating that the amorphous RuO2/AZO has the poorest surface properties among all of the synthesized samples. In general, a high surface area and a large pore volume are favorable, as a high internal surface area can provide numerous reaction sites for oxidation and reduction, a condition which is advantageous for increasing the specific capacity. Moreover, a large pore volume can reduce the blockage of the available pores caused by the deposition of ORR products, resulting in improved electrochemical performance.8 In addition, many researchers have reported that the electrical conductivity of AZO is highly dependent on the annealing temperature, and that with an increase in the annealing temperature, the electrical conductivity can be improved at temperatures up to 500 °C. Judging by these factors, annealing materials at low temperatures would not be preferred for cathode materials. However, the electrochemical properties of RuO2/AZO were contrary to our expectations. The voltage profiles of amorphous and crystalline RuO2/AZO prepared at diverse temperature are shown in Fig. 3. The amorphous RuO2/AZO shows improved performance compared to the crystalline RuO2/AZO, exhibiting a specific capacity of 1040 mA h g−1 and an overpotential of 0.8 V. With an increase in the annealing temperature, the electrochemical performance levels of RuO2/AZO deteriorate. The specific capacity of the crystalline RuO2/AZO prepared at 500 °C dropped significantly to 830 mA h g−1 with an increment in the overpotential of 1.0 V. Despite the fact that the specific capacity and discharge overpotential of the crystalline RuO2/AZO annealed at 400 °C are equal to those of the amorphous RuO2/AZO, the charge overpotential is much higher, resulting in a total overpotential of 1.2 V. This is because the catalytic activity of amorphous RuO2 toward OER is higher than that of crystalline RuO2. Consequently, it can be concluded that amorphous RuO2/AZO offers better electrochemical properties compared to those of crystalline RuO2/AZO, despite the fact that the properties of AZO, such as the BET surface properties and electrical conductivity, cannot meet the required specifications. This countertrend can be explained by the use of the electrocatalyst RuO2. It can be deduced that the catalytic activity of RuO2 is associated with crystallinity of the material. The catalytic activity of amorphous RuO2 is higher than that of crystalline RuO2, and it diminishes with an increase in the crystallinity. Therefore, it can be assumed that in certain materials such as AZO, the electrochemical properties of Li–O2 batteries are strongly influenced by the electrocatalyst and the catalytic effect is dominant over the properties of the cathode materials.
The reversible formation and decomposition of the oxygen reduction reaction (ORR) products on the surface of the amorphous RuO2/AZO were investigated by field-emission scanning electron microscopy (FE-SEM) and by an X-ray photoelectron spectroscopy (XPS) analysis. Fig. 4 shows FE-SEM images of the as-prepared amorphous RuO2/AZO, after the first discharge and after the first charge. Each electrode was washed with dimethyl ether (DME) in order to remove any residual Li salt from the electrolyte. As shown in Fig. 4(b), ORR products were deposited onto the surface of the amorphous RuO2/AZO after a full discharge, as confirmed by the Li 1s and O 1s XPS spectra shown in Fig. S2.† A binding energy peak of about 55 eV in the Li 1s spectrum and 531.5 eV in the O 1s spectrum represent the presence of Li2O2. A reversible reaction was observed, in that the peak corresponding to Li2O2 disappeared in the Li 1s and O 1s spectra after the first charge. This is strongly supported by the FE-SEM image in Fig. 4(c), in which all of the ORR products deposited onto the surface of the cathode materials were completely decomposed after the first charge. Therefore, amorphous RuO2/AZO is electrochemically active toward the formation and decomposition of Li2O2 on the cathode surface, which is a fundamental redox reaction in Li–O2 batteries. Moreover, the reaction is reversible.
 | ||
| Fig. 4 FE-SEM images of amorphous RuO2/AZO: (a) pristine (b) after the first discharge (c) after the first charge. | ||
Conclusion
In summary, amorphous RuO2/AZO was synthesized by a microwave-assisted hydrothermal method and developed as a carbon-free cathode material for Li–O2 batteries for the first time. The composite of the electrocatalyst RuO2 and cathode material of AZO was fabricated in an in situ synthesis, which offers a simple, rapid, and cost-effective method, as additional steps are not needed. The crystallinity of RuO2 has a strong effect on the catalytic activity in Li–O2 batteries. The catalytic activity of RuO2 was increased in conjunction with decreased crystallinity, indicating that amorphous RuO2 can be considered to be the most effective electrocatalyst with AZO. As a carbon-free cathode with RuO2/AZO, the electrochemical properties were significantly affected by RuO2 as compared to AZO, signifying that the catalytic activity has a dominant effect on Li–O2 batteries with certain materials, such as AZO in this case. Further studies are necessary to clarify the role of crystallinity in RuO2.Acknowledgements
This research was supported by the Government-Funded General Research & Development Program by the Ministry of Trade, Industry and Energy, Korea.Notes and references
- Y.-C. Lu, H. A. Gasteiger and Y. Shao-Horn, J. Am. Chem. Soc., 2011, 133, 19048 CrossRef CAS PubMed.
- G. Girishkumar, B. McCloskey, A. C. Luntz, S. Swanson and W. Wilcke, J. Phys. Chem. Lett., 2010, 1, 2193 CrossRef CAS; R. R. Mitchell, B. M. Gallant, C. V. Thompson and Y. S. Horn, Energy Environ. Sci., 2011, 4, 2952 Search PubMed; K. Guo, Y. Li, J. Yang, Z. Zou, X. Xue, X. Li and H. Yang, J. Mater. Chem., 2014, 2, 1509 RSC.
- A. Riaz, K.-N. Jung, W. Chang, S.-B. Lee, T.-H. Lim, S.-J. Park, R. H. Song, S. Yoon, K.-H. Shin and J.-W. Lee, Chem. Commun., 2013, 49, 5984 RSC; B. D. McCloskey, A. Speidel, R. Scheffler, D. C. Miller, V. Viswanathan, J. S. Hummelshoj, J. K. Norskov and A. C. Luntz, J. Phys. Chem. Lett., 2012, 3, 997 CrossRef CAS; F. Li, H. Kitaura and H. Zhou, Energy Environ. Sci., 2013, 6, 2302 Search PubMed.
- S. A. Freunberger, Y. Chen, Z. Peng, J. M. Griffin, L. J. Hardwick, F. Barde, P. Novak and P. G. Bruce, J. Am. Chem. Soc., 2011, 133, 8040 CrossRef CAS PubMed; J. Hong, H.-D. Lim, M. Lee, S.-W. Kim, H. Kim, S.-T. Oh, G.-C. Chung and K. Kang, Chem. Mater., 2012, 24, 2692 CrossRef; W. Xu, K. Xu, V. V. Viswanathan, S. A. Towne, J. S. Hardy, J. Xiao, Z. Nie, D. Hu, D. Wang and J.-G. Zhang, J. Power Sources, 2011, 196, 9631 CrossRef PubMed; B. D. McCloskey, D. S. Bethune, R. M. Shelby, G. Girishkumar and A. C. Luntz, J. Phys. Chem. Lett., 2011, 2, 1161 CrossRef.
- F. Li, D.-M. Tang, Y. Chen, D. Golberg, H. Kitaura, T. Zhang, A. Yamada and H. Zhou, Nano Lett., 2013, 13, 4702 CrossRef CAS PubMed.
- H.-M. Zhou, D.-Q. Yi, Z.-M. Yu, L.-R. Xiao and J. Li, Thin Solid Films, 2007, 515, 6909 CrossRef CAS PubMed.
- L. Wang, Y. Huang, R. Jiang and D. Jia, Electrochim. Acta, 2007, 52, 6778 CrossRef CAS PubMed; I. Bilecka and M. Niederberger, Nanoscale, 2010, 2, 1358 RSC.
- W. Zhang, Y. Zeng, C. Xu, H. Tan, W. Liu, J. Zhu, N. Xiao, H. H. Hng, J. Ma, H. E. Hoster, R. Yazami and Q. Yan, RSC Adv., 2012, 2, 8508 RSC; H. Cheng and K. Scott, J. Power Sources, 2010, 195, 1370 CrossRef CAS PubMed; J. Xiao, D. Wang, W. Xu, D. Wang, R. E. Williford, J. Liu and J.-G. Zhang, J. Electrochem. Soc., 2010, 157, A487 CrossRef PubMed; S. S. Zhang, D. Foster and J. Read, J. Power Sources, 2010, 195, 1235 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, TEM images, XPS analysis, BET analysis. See DOI: 10.1039/c4ra16612d |
| This journal is © The Royal Society of Chemistry 2015 |