Enhanced visible-light-driven photoactivities of single-walled carbon nanotubes coated with N doped TiO2 nanoparticles
Tianyu Gaoa,
Guocheng Suna,
Feiyue Chenga,
Ke Dai*a,
Hao Chen*b,
Kejian Dengc and
Qiaoyun Huanga
aCollege of Resources and Environment, Huazhong Agricultural University, Wuhan 430070, P. R. China. E-mail: dk@mail.hzau.edu.cn; Fax: +86-27-8728-2137; Tel: +86-27-8767-1033
bCollege of Science, Huazhong Agricultural University, Wuhan 430070, P. R. China. E-mail: hchenhao@mail.hzau.edu.cn
cKey Laboratory of Catalysis and Materials Science of the State South-Central University for Nationalities, Wuhan 430074, P. R. China
First published on 18th March 2015
Abstract
A two-step hydrothermal method is used to prepare N doped single-walled carbon nanotube–TiO2 (SWCNT–N/TiO2) hybrids with different contents of SWCNTs from 1.25 wt% to 10 wt%. The photocatalysts were characterized by X-ray diffraction analysis, UV-vis diffuse reflectance spectroscopy, Raman spectroscopy, scanning electron microscopy, transmission electron microscopy, and X-ray photoelectron spectroscopy. The UV-vis diffuse reflectance spectra show an apparent enhancement of absorption throughout the visible light region. Raman spectroscopy further confirms the chemical interaction between N/TiO2 and SWCNTs in the hybrid. SWCNT–N/TiO2 hybrid presents a superior photocatalytic activity to DWCNT–N/TiO2 (N doped double-walled carbon nanotube–TiO2) and MWCNT–N/TiO2 (N doped multi-walled carbon nanotube–TiO2) based on the results of the photocatalytic degradation of sulfathiazole under visible light irradiation, which is attributed to the synergetic effects of SWCNT modification and N doping. N doping induces visible light absorption by either introducing localized electronic states within the band gap or contributing electrons to the valence band and metal-like SWCNT can act as an electron conductor to facilitate the fast injection of the photogenerated electrons into the conduction band of N/TiO2, leading to significantly enhanced visible-light-driven photocatalytic activity.
1. Introduction
Photocatalysis is a potential technology in meeting cleaning energy demands and environmental remediation.1,2 Recently, many researchers have applied photocatalysis to remove organic pollutants in aqueous suspension and demonstrated that the photocatalyst displayed a good removal effect on those pollutants.3–5 By far, TiO2 has emerged as the most promising photocatalyst because of its high oxidative power, photostability, low cost, and nontoxicity. However, the band gap energy (Eg = 3.2 eV) of the anatase TiO2 is a major disadvantage for its application, since the photocatalytic process can only be initiated under UV irradiation (which is only approximately 4% of solar radiation). Moreover, the low quantum efficiency caused by fast recombination of photogenerated h+–e− pairs is another drawback when TiO2 is used to be a photocatalyst. In overcoming the above disadvantages, modification on TiO2 has drawn a lot of attention from researchers. Doping with metal/nonmetal elements and surface modification with semiconductor of narrow band-gap are both typical approaches to generate visible-light-driven photoactivity from TiO2. Among the nonmetal dopants, nitrogen has been considered as an effective dopant since N doped TiO2 (N/TiO2) was synthesized firstly by Asahi et al.6 N/TiO2 has a new energy band gap lower than pristine TiO2 owing to the mixing of the 2p nitrogen level with the oxygen 2p orbital to form the valence band. Consequently, the rate of recombination of h+–e− pairs is higher, which remains a serious obstacle to achieving high visible-light-induced photocatalytic activity. Several studies have investigated techniques to reduce the electron–hole combination rate via metal coating/doping.7,8 Noble metal nanoparticles can effectively enhance the charge carrier separation by forming a Schottky barrier on the surface of TiO2. In addition, generation of surface plasmon resonance can help to shift the absorption of the TiO2 in the visible region.8In the recent years, carbon nanotubes (CNTs) has received growingly considerable attention on the field of photocatalysis due to its unique electronic and physical properties,9,10 which could act as an electron acceptor because of the conductive structure of CNTs scaffolds and hence induced efficient charge transfer.11,12 It is expected that CNTs may also act as effective electron sinks because of its high electrical conductivity and electron storage capacity which was demonstrated by Yu et al.13,14 Wang et al.15 argued that CNTs was utilized to be a photosensitizer which can enhance the visible-light-driven photocatalytic activity of TiO2. However, we reported the functionalized single-walled carbon nanotubes (SWCNTs) coated with TiO2 nanoparticles via hydrothermally method where SWCNTs act as an electrical conductor rather than photosensitizer, achieving the improved photoactivity.16 Although, studies always focused more on the multi-walled carbon nanotube–TiO2 (MWCNT–TiO2) than SWCNT–TiO2,13,17,18 SWCNTs possess a high specific surface area and provides better catalyst-support (dispersal and connection) than MWCNTs, which can achieve a better understanding of the proposed mechanisms.19 As a novel material, SWCNT–TiO2 has been fabricated by various ways.20 Zhou et al.21 prepared SWCNT–TiO2 by a facile sol–solvothermal method suggesting that TiO2 and SWNCTs linked compactly through ester bonds and thus improved their interfaces which could diminish rate of h+–e− pair's recombination. Vajda et al.22 synthesized SWCNT–TiO2 by the aid of ultrasonication method and got the optimal composition of the SWCNT–TiO2 for the degradation of phenol under UV irradiation. However, the enhancement on the photoactivity of TiO2 after the introduction of SWCNTs is limited under visible light irradiation and the modification of SWCNT–TiO2 using nitrogen anion to promote photocatalytic activity in the visible light region is less reported.
In this study, we synthesized for the first time a series of SWCNT–N/TiO2 hybrid by directly growing TiO2 on the surface of SWCNTs with hydrothermal treatment and then substantial calcination using an N source. The effects of the type of the CNTs (SWCNTs, MWCNTs, and double-walled carbon nanotubes (DWCNTs)) and the contact between CNTs and N/TiO2 on the visible-light-responsive photoactivity of the CNT–N/TiO2 hybrids were extensively investigated according to the experimental results of photocatalytic degradation of sulfathiazole under visible light irradiation.
2. Experimental
2.1 Materials
SWCNTs were purchased from Chengdu Organic Chemicals (CAS, China). All other reagents, including Titanium sulfate (Ti(SO4)2), cetyltrimethylammonium bromide (CTAB), sodium chloride, ethanol, nitric acid and urea were commercially available in analytical purity and used as received.2.2 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), heat stirred for 2 h, followed by rinsing with deionized water until pH value of supernatant became neutral. Next, the dissolved Ti(SO4)2 was added to the functionalized SWCNTs, CTAB and deionized water (m(Ti(SO4)2)
1), heat stirred for 2 h, followed by rinsing with deionized water until pH value of supernatant became neutral. Next, the dissolved Ti(SO4)2 was added to the functionalized SWCNTs, CTAB and deionized water (m(Ti(SO4)2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m(CTAB)
m(CTAB)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m(water) = 1
m(water) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12
0.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100). After stirring for 30 min, the resulting mixture was decanted into a 50 mL stainless steel autoclave for 72 h of hydrothermal treatment at 100 °C. When the suspension cooled down to room temperature, it was centrifuged and rinsed with deionized water and ethanol for 2–3 times. In order to remove the CTAB, ion-exchange treatment was performed by mixing the obtained material with a water and ethanol (molar ratio 1
100). After stirring for 30 min, the resulting mixture was decanted into a 50 mL stainless steel autoclave for 72 h of hydrothermal treatment at 100 °C. When the suspension cooled down to room temperature, it was centrifuged and rinsed with deionized water and ethanol for 2–3 times. In order to remove the CTAB, ion-exchange treatment was performed by mixing the obtained material with a water and ethanol (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution of sodium chloride under stirring at ambient temperature for 24 h, then dried at 80 °C overnight. At last, the obtained powder was calcined at 400 °C for 2 h in Muffle furnace.
1) solution of sodium chloride under stirring at ambient temperature for 24 h, then dried at 80 °C overnight. At last, the obtained powder was calcined at 400 °C for 2 h in Muffle furnace.The preparation process for the 2.5 wt% SWCNT–N/TiO2 goes as follows: 0.5 g of SWCNT–TiO2 and 3.5 g of urea were dispersed in 3 mL deionized water and 1 mL ethanol. The mixture was stirred for 1 h, dried at 80 °C to jelly, and finally calcined at 450 °C for 2 h to obtain SWCNT–N/TiO2 hybrid. The contents of SWCNTs in the hybrid were controlled at 1.25, 2.5, 5.0, and 10.0 wt%.
3. Results and discussion
3.1 Structure and morphology of the SWCNT–N/TiO2 hybrid
The effects of N doping and SWCNTs modification on TiO2 crystallization was investigated by XRD (shown in Fig. 1). The diffraction peaks at 2θ values of 25.33°, 37.91°, 48.03°, 54.47°, 55.0°, 62.97° can be indexed to the (101), (004), (200), (105), (211) and (204) planes of anatase phase, respectively.23 This phenomenon may indicate that dopant did not alter the crystallite structure of TiO2 but nitrogen anions have moved into either the interstitial positions (Ti–O–N) or the substitutional sites (O–Ti–N) of the TiO2 crystal structure.24,25 In addition, the characteristic peaks of SWCNTs at 26.6° and 43.5° are not detected in the 1.25 wt% to 10 wt% SWCNT–N/TiO2 hybrids, which can be attributed to the good dispersion of SWCNTs in the hybrid after surface functionalization.16 The primary crystallite size of N/TiO2 and 1.25 wt% to 10 wt% SWCNT–N/TiO2 hybrids (presented in Table 1) was calculated by the Scherrer equation:d = (0.89 × λ)/β × cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
 | ||
| Fig. 1 X-ray diffraction patterns of TiO2, N/TiO2, 5 wt% SWCNT–TiO2 and SWCNT–N/TiO2 hybrids with different contents of SWCNTs. | ||
| Samples | Crystallite size (nm) | λ (nm) | Eg (eV) |
|---|---|---|---|
| TiO2 | 18.1[16] | 400 | 3.1 |
| 5 wt% SWCNT–TiO2 | 11.2[16] | 428 | 2.90 |
| N/TiO2 | 9.6 | 530 | 2.34 |
| 1.25 wt% SWCNT–N/TiO2 | 14.6 | 449 | 2.76 |
| 2.5 wt% SWCNT–N/TiO2 | 10.2 | 561 | 2.21 |
| 5 wt% SWCNT–N/TiO2 | 11.4 | 432 | 2.87 |
| 10 wt% SWCNT–N/TiO2 | 10.1 | 504 | 2.40 |
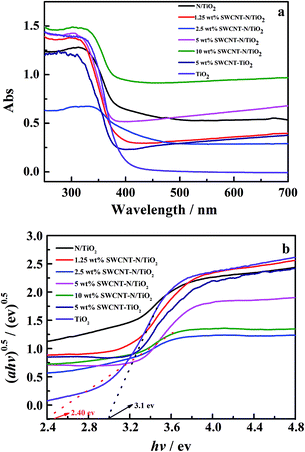
The DRS spectra of TiO2, N/TiO2, SWCNT–TiO2, and SWCNT–N/TiO2 with different contents of SWCNTs are shown in Fig. 2a. The spectrum for TiO2 has an absorption edge at 400 nm, corresponding to a band-gap energy of about 3.1 eV. Compared with pure TiO2, N/TiO2, SWCNT–TiO2 and SWCNT–N/TiO2 hybrids contain less TiO2, which leads to a weaker absorption in the UV region. Although the absorption thresholds of SWCNT–N/TiO2 hybrids are near to that of TiO2, all SWCNT–N/TiO2 hybrids have better absorption in the visible light region (>400 nm). Compared with the 5 wt% SWCNT–TiO2, a greater adsorption property of 5 wt% SWCNT–N/TiO2 hybrid is obtained from the DRS spectra. This phenomenon may demonstrate that nitrogen anions substituted for oxygen positions in the TiO2 lattice, thereby forming a mid-gap level between the valence and conduction bands of TiO2.25
The band gap energy of TiO2, N/TiO2, SWCNT–TiO2, SWCNT–N/TiO2 hybrids were calculated by plotting (αhν)0.5 versus hν where α is the absorption coefficient (Fig. 2b). The results suggest that both SWCNT modification and N doping could narrow the band gap of TiO2, which lead to a broader optical absorption range; however, the band gap initially narrow and then enlarge with the increase of loading amount of SWCNTs (shown in Table 1), this conclusion indicates that whether the decrease in band gap enhances photocatalytic activity or not depends on the wavelength of incident light.26
Fig. 3 shows the SEM and TEM images of 2.5 wt% SWCNT–TiO2 and 2.5 wt% SWCNT–N/TiO2. As shown in Fig. 3a and b, the acid-treated SWCNT morphology remain the original structure and is densely covered with a large amount of TiO2 or N/TiO2 regardless of nitrogen doping or not, suggesting that TiO2 or N/TiO2 was coated on the outer surface of the SWCNTs according to the field emission theory.27 On the other hand, the N/TiO2 nanoparticles are homogeneously distributed on the surface of acid-treated SWCNTs (Fig. 3c) owing to the effect of surface functional groups like carboxyl and hydroxyl, which can contribute to a high specific surface area.28 Meanwhile, lattice fringes can be clearly seen in TEM image (Fig. 3d) indicating that the d spacing for TiO2 (101) planes is 0.35 nm consistent with XRD results. Close interfacial contact between N/TiO2 and the SWCNTs is observed, which is advantageous for transferring electrons and retarding the recombination of photogenerated h+–e− pairs, thus improving the photocatalytic activity.21
 | ||
| Fig. 3 SEM images of the 2.5 wt% SWCNT–TiO2 (a) and 2.5 wt% SWCNT–N/TiO2 (b); TEM images of the 2.5 wt% SWCNT–N/TiO2 (c and d). | ||
Raman spectrum of TiO2, SWCNTs, 2.5 wt% SWCNT–TiO2, and 2.5 wt% SWCNT–N/TiO2 are presented in Fig. 4. The characteristic bands at 402.1, 517.4, 636.2 cm−1 in the 2.5 wt% SWCNT–N/TiO2 hybrid corresponding to B1g, A1g, Eg modes of anatase, respectively; no Raman peak due to TiN can be observed in the 2.5 wt% SWCNT–N/TiO2 hybrid which is in agreement with XRD pattern. Furthermore, our results show an obvious red-shift for the Eg mode of the 2.5 wt% SWCNT–N/TiO2 hybrid and 5 wt% SWCNT–TiO2, indicating a decrease nanoparticle size according to the report of Zhang et al.29 However, compared with 5 wt% SWCNT–TiO2, the Raman peaks of 2.5 wt% SWCNT–N/TiO2 hybrid shift to lower frequency due to lattice expansion, which confirm the presence of the dopant anion in the crystal lattice.25 The characteristic D-band (disordered mode) at 1353 cm−1 and G-band (tangential mode) at 1580 cm−1 of SWCNTs could be observed, indicating the existence of SWCNTs in the hybrid. The relative intensity ratio of the D-band to the G-band is known as an index of graphitization to determine the CNTs microstructure.30 The ID/IG ratio of the hybrid is 0.11 similar to the pure SWCNTs,16 which demonstrates N doping and hydrothermal treatment did not damage the graphitization structure of the 2.5 wt% SWCNT–N/TiO2 hybrid.
XPS measurements were carried out to further determine the interaction of SWCNTs and N/TiO2 nanoparticles. Fig. 5a presents C 1s, O 1s, Ti 2p, Ti 3s, Ti 3p and N 1s XPS spectrum of 5 wt% SWCNT–N/TiO2 hybrid. It is observed that C 1s spectrum (Fig. 5b) can be fitted to the three peaks, located at 284.6, 285.7, 288.6 eV, which are ascribed to C–C, C–O, and C–O–O bonds, respectively. The existence of these polar groups demonstrates that the surface of SWCNTs was oxidized to some extent, which is beneficial for the adsorption of the precursor molecules and the nucleation of N/TiO2 on the surface of SWCNTs.31 Fig. 5c displays the XPS spectrum of Ti 2p for the 5 wt% SWCNT–N/TiO2 hybrid, which indicates the two peaks at 458.9 and 464.8 eV corresponding to the photo-splitting electrons Ti 2p3/2 and Ti 2p1/2, respectively. The binding energies of two peaks presented a blue-shift compared to the CNT/TiO2,32 which is attributed to the substitution of a less electronegative nitrogen atom in the place of oxygen in spite of the strong interaction existed between N/TiO2 nanoparticles and SWCNTs.31 Furthermore, the O 1s peak for the hybrid appears at 530.0 eV (Fig. 5d), suggesting the presence of the substitutional N (O–Ti–N) rather than interstitial N (Ti–O–N).25 The N 1s spectrum of the hybrid has a main peak at 399.0 eV (Fig. 5e), which is greater than the typical binding energy of 396.9 eV in TiN,33 which can be attributed to the 1s electron binding energy of the N atom in the environment of O–Ti–N. This shift to a higher energy is ascribed to the formation of O–Ti–N structure when nitrogen substitutes for oxygen in TiO2, and hence the electron density around nitrogen is less than in TiN.25
 | ||
| Fig. 5 XPS spectra for the SWCNT–N/TiO2 hybrid: survey (a), C 1s (b), Ti 2p (c), O 1s (d), and N 1s (e). | ||
3.2 Photocatalytic activity
To investigate the contact between the SWCNTs and N/TiO2 on the visible-light-induced photoactivity of the SWCNT–N/TiO2 hybrid, homemade TiO2, N/TiO2, 5 wt% SWCNT + N/TiO2 (a simple mixture of SWCNTs and N/TiO2 at a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19) and a series of SWCNT–N/TiO2 hybrids synthesized by the hydrothermal method were examined in a solution of sulfathiazole under visible light irradiation. The results of degradation efficiency are illustrated in Fig. 6. It is obvious that sulfathiazole was barely decomposed in the absence of photocatalyst. This phenomenon indicates that sulfathiazole elimination by the hydrolysis and/or photolysis can be neglected under visible light irradiation. Photocatalytic degradation of sulfathiazole over homemade TiO2 was limited because of the low photoactivity of TiO2 under visible light.34 Simultaneously, the enhancement on the photoactivity of TiO2 after the introduction of SWCNTs was inconspicuous. According to Zhu et al.,28 a metal possesses a higher work function than that of the n-type semiconductor (such as TiO2), electrons can flow from the semiconductor into the metal for adjusting the Fermi energy levels. In general, as-synthesized SWCNTs are composed of one-third metal and two-thirds semiconductor ones; therefore SWCNTs cannot act as photosensitizer to improve the visible-light-induced photoactivity.35 Nevertheless, N/TiO2 exhibits a high photoactivity, since substitutional nitrogen forms new state that lies just above the valence band of TiO2 which causes band gap narrowing enabling it to absorb visible light.36 Moreover, the 5 wt% SWCNT + N/TiO2 showed a slower removal rate of sulfathiazole than the 5 wt% SWCNT–N/TiO2 hybrid. According to these experimental results, a possible explanation for the higher photoactivity of 5 wt% SWCNT–N/TiO2 hybrid is the intimate interaction of N/TiO2 with the SWCNTs and the formation of localized electronic states in the band gap caused by N doping.37 In our study, the 5 wt% SWCNT–N/TiO2 hybrid presented superior photoactivity to 5 wt% DWCNT–N/TiO2 hybrid and 5 wt% MWCNT–N/TiO2 hybrid (Fig. 6). The phenomenon is different from the previous study, according to the report of Liu et al.10 SWCNT–TiO2 presented less photoactivity than DWCNT–TiO2 and MWCNT–TiO2. As we know, the structure of CNTs plays an important role in the photocatalytic process. SWCNTs' architecture provides a network to disperse N/TiO2 nanoparticles. An increase of photoconversion efficiency represents the beneficial role of the SWCNTs as conducting scaffold to facilitate the fast injection of the photogenerated electrons on the CB of N/TiO2.38 Furthermore, the photocatalytic activity of the hybrids with varying SWCNTs content was also investigated. As shown in Fig. 7 and 5 wt% SWCNT–N/TiO2 demonstrated the highest photoactivity with a degradation rate of approximately 100% after reaction of 1 h. By contrast, the effect of 10 wt% SWCNT–N/TiO2 was not favorable since the excessive SWCNTs can act as recombination center for h+–e− pairs, resulting in depressed photoactivity.16
19) and a series of SWCNT–N/TiO2 hybrids synthesized by the hydrothermal method were examined in a solution of sulfathiazole under visible light irradiation. The results of degradation efficiency are illustrated in Fig. 6. It is obvious that sulfathiazole was barely decomposed in the absence of photocatalyst. This phenomenon indicates that sulfathiazole elimination by the hydrolysis and/or photolysis can be neglected under visible light irradiation. Photocatalytic degradation of sulfathiazole over homemade TiO2 was limited because of the low photoactivity of TiO2 under visible light.34 Simultaneously, the enhancement on the photoactivity of TiO2 after the introduction of SWCNTs was inconspicuous. According to Zhu et al.,28 a metal possesses a higher work function than that of the n-type semiconductor (such as TiO2), electrons can flow from the semiconductor into the metal for adjusting the Fermi energy levels. In general, as-synthesized SWCNTs are composed of one-third metal and two-thirds semiconductor ones; therefore SWCNTs cannot act as photosensitizer to improve the visible-light-induced photoactivity.35 Nevertheless, N/TiO2 exhibits a high photoactivity, since substitutional nitrogen forms new state that lies just above the valence band of TiO2 which causes band gap narrowing enabling it to absorb visible light.36 Moreover, the 5 wt% SWCNT + N/TiO2 showed a slower removal rate of sulfathiazole than the 5 wt% SWCNT–N/TiO2 hybrid. According to these experimental results, a possible explanation for the higher photoactivity of 5 wt% SWCNT–N/TiO2 hybrid is the intimate interaction of N/TiO2 with the SWCNTs and the formation of localized electronic states in the band gap caused by N doping.37 In our study, the 5 wt% SWCNT–N/TiO2 hybrid presented superior photoactivity to 5 wt% DWCNT–N/TiO2 hybrid and 5 wt% MWCNT–N/TiO2 hybrid (Fig. 6). The phenomenon is different from the previous study, according to the report of Liu et al.10 SWCNT–TiO2 presented less photoactivity than DWCNT–TiO2 and MWCNT–TiO2. As we know, the structure of CNTs plays an important role in the photocatalytic process. SWCNTs' architecture provides a network to disperse N/TiO2 nanoparticles. An increase of photoconversion efficiency represents the beneficial role of the SWCNTs as conducting scaffold to facilitate the fast injection of the photogenerated electrons on the CB of N/TiO2.38 Furthermore, the photocatalytic activity of the hybrids with varying SWCNTs content was also investigated. As shown in Fig. 7 and 5 wt% SWCNT–N/TiO2 demonstrated the highest photoactivity with a degradation rate of approximately 100% after reaction of 1 h. By contrast, the effect of 10 wt% SWCNT–N/TiO2 was not favorable since the excessive SWCNTs can act as recombination center for h+–e− pairs, resulting in depressed photoactivity.16
Based on the experimental findings and the discussion above, the proposed mechanism for the enhanced photoactivity of SWCNT–N/TiO2 hybrid is summarized (Fig. 8). N/TiO2 exhibits a relatively lower energy band gap than that of pure TiO2 for electrons to emit from the up-shifting Fermi level into the SWCNT, which results in the metal-like SWCNT possessing an excess negative charge and the VB of semiconductor (N/TiO2) an excess positive charge.27 Therefore, SWCNT–N/TiO2 hybrid can form a semiconductor–metal junction called a Schottky barrier which offers an effective route of decreasing recombination of photogenerated h+–e− pairs.29 The electrons on SWCNTs can further generate superoxide radical from oxygen. Simultaneously, the produced holes can be transferred from the valence band to the surface of N/TiO2 where they can directly oxidize organic pollutants or indirectly oxidize them through the formation of hydroxyl radicals.15,39
 | ||
| Fig. 8 Proposed mechanism for photocatalytic degradation of sulfathiazole over the SWCNT–N/TiO2 hybrid; S: sulfathiazole; Sox: oxidized sulfathiazole. | ||
4. Conclusions
N doped and single-walled carbon nanotube modified TiO2 (SWCNT–N/TiO2) hybrid was synthesized via a two-step hydrothermal method and analyzed using XRD, DRS, SEM, TEM, Raman, and XPS techniques. SWCNTs in the hybrid were coated with N/TiO2 nanoparticles, which decrease the crystallization degree of the anatase and generate an intimate contact between the SWCNTs and N/TiO2. Compared with N/TiO2 and SWCNT–TiO2, the high visible-light-induced photoactivity can be rationalized by the synergetic effects originated from both SWCNT modification and N doping. Moreover, SWCNT–N/TiO2 hybrid showed better photocatalytic activity than DWCNT–N/TiO2 and MWCNT–N/TiO2 in the photocatalytic degradation of sulfathiazole under visible light irradiation. The experimental results demonstrate that the SWCNTs act more as an electrical conductor than a photosensitizer, which efficiently suppress charge recombination, resulting in improving interfacial charge transfer, and eventually enhance the photoactivity of SWCNT–N/TiO2 hybrid.Acknowledgements
The research was financially supported by the National Natural Science Foundation of China (21307035), Open Foundation of Key Laboratory of Catalysis and Materials Science of the State Ethnic Affairs Commission and Ministry of Education, South-Central University for Nationalities (CHCL12004), Fundamental Research Funds for Central Universities of China (2013PY112), and the National Training Programs of Innovation and Entrepreneurship for Undergraduates (201210504130).References
- F. Wang, W. G. Wang, X. J. Wang, H. Y. Wang, C. H. Tung and L. Z. Wu, Angew. Chem., Int. Ed., 2011, 50, 3193–3197 CrossRef CAS PubMed.
- M. Elvington, J. Brown, S. M. Arachchige and K. J. Brewer, J. Am. Chem. Soc., 2007, 129, 10644–11645 CrossRef CAS PubMed.
- M. N. Abellan, B. Bayarri, J. Gimenez and J. Costa, Appl. Catal., B, 2007, 74, 233–241 CrossRef CAS PubMed.
- M. N. Abellan, J. Gimenez and S. Esplugas, Catal. Today, 2009, 144, 131–136 CrossRef CAS PubMed.
- W. Baran, J. Sochacka and W. Wardas, Chemosphere, 2006, 65, 1295–1299 CrossRef CAS PubMed.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- S. S. Zhang, F. Peng, H. J. Wang, H. Yu, S. Q. Zhang, J. A. Yang and H. J. Zhao, Catal. Commun., 2011, 12, 689–693 CrossRef CAS PubMed.
- R. S. Dhabbe, A. N. Kadam, M. B. Suwarnkar, M. R. Kokate and K. M. Garadkar, J. Mater. Sci.: Mater. Electron., 2014, 25, 3179–3189 CrossRef CAS PubMed.
- H. P. Yang, S. Wu, Y. P. Duan, X. F. Fu and J. M. Wu, Appl. Surf. Sci., 2011, 258, 3012–3018 CrossRef PubMed.
- C. W. Liu, H. B. Chen, K. Dai, A. F. Xue, H. Chen and Q. Y. Huang, Mater. Res. Bull., 2013, 48, 1499–1505 CrossRef CAS PubMed.
- H. Wang, H. L. Wang, W. F. Jiang and Z. Q. Li, Water Res., 2009, 43, 204–210 CrossRef CAS PubMed.
- A. Jitianu, T. Cacciaguerra, R. Benoit, S. Delpeux, F. Beguin and S. Bonnamy, Carbon, 2004, 42, 1147–1151 CrossRef CAS PubMed.
- Y. Yu, J. C. Yu, C. Y. Chan, Y. K. Che, J. C. Zhao and L. Ding, Appl. Catal., B, 2005, 61, 1–11 CrossRef CAS PubMed.
- T. A. Saleh and V. K. Gupta, J. Colloid Interface Sci., 2011, 362, 337–344 CrossRef CAS PubMed.
- W. D. Wang, P. Serp, P. Kalck and J. L. Faria, J. Mol. Catal. A: Chem., 2005, 235, 194–199 CrossRef CAS PubMed.
- K. Dai, X. H. Zhang, K. Fan, T. Y. Peng and B. Q. Wei, Appl. Surf. Sci., 2013, 270, 238–244 CrossRef CAS PubMed.
- K. Dai, T. Y. Peng, D. N. Ke and B. Q. Wei, Nanotechnology, 2009, 20, 125603 CrossRef PubMed.
- K. Woan, G. Pyrgiotakis and W. Sigmund, Adv. Mater., 2009, 21, 2233–2239 CrossRef CAS.
- Y. Yao, G. Li, S. Ciston, R. M. Lueptow and K. A. Gray, Environ. Sci. Technol., 2008, 42, 4952–4957 CrossRef CAS.
- H. R. Jafry, M. V. Liga, Q. L. Li and A. R. Barron, New J. Chem., 2011, 35, 400–406 RSC.
- W. Zhou, K. Pan, Y. Qu, F. F. Sun, C. G. Tian, Z. Y. Ren, G. H. Tian and H. G. Fu, Chemosphere, 2010, 81, 555–561 CrossRef CAS PubMed.
- K. Vajda, K. Mogyorosi, Z. Nemeth, K. Hernadi, L. Forro, A. Magrez and A. Dombi, Phys. Status Solidi B, 2011, 248, 2496–2499 CrossRef CAS.
- G. H. Jiang, X. Y. Zheng, Y. Wang, T. W. Li and X. K. Sun, Powder Technol., 2011, 207, 465–469 CrossRef CAS PubMed.
- N. Patel, R. Jaiswal, T. Warang, G. Scarduelli, A. Dashora, B. L. Ahuja, D. C. Kothari and A. Miotello, Appl. Catal., B, 2014, 150–151, 74–81 CrossRef CAS PubMed.
- Y. Gurkan, N. Turkten, A. Hatipoglu and Z. Cinar, Chem. Eng. J., 2012, 184, 113–124 CrossRef CAS PubMed.
- H. Chen, S. Yang, K. Yu, Y. M. Yu and C. Sun, J. Phys. Chem. A, 2011, 115, 3034–3041 CrossRef CAS PubMed.
- P. H. Chen, Y. S. Huang, W. J. Su, K. Y. Lee and K. K. Toing, Mater. Chem. Phys., 2014, 143, 1378–1383 CrossRef CAS PubMed.
- S. H. Wang, L. J. Ji, B. Wu, Q. M. Gong, Y. F. Zhu and J. Liang, Appl. Surf. Sci., 2008, 255, 3263–3266 CrossRef CAS PubMed.
- L. W. Zhang, H. B. Fu and Y. F. Zhu, Adv. Funct. Mater., 2008, 18, 2180–2189 CrossRef CAS.
- H. M. Heise, R. Kuckuk, A. K. Ojha, A. Srivastava, V. Srivastava and B. P. Asthana, J. Raman Spectrosc., 2009, 40, 344–353 CrossRef CAS.
- S. H. Liu and H. R. Syu, Appl. Energy, 2012, 100, 148–154 CrossRef CAS PubMed.
- G. M. An, W. H. Ma, Z. Y. Sun, Z. M. Liu, B. X. Han, S. D. Miao, Z. J. Miao and K. L. Ding, Carbon, 2007, 45, 1795–1801 CrossRef CAS PubMed.
- A. V. Emeline, V. N. Kuznetsov, V. K. Rybchuk and N. Serpone, Int. J. Photoenergy, 2008, 258394 Search PubMed.
- T. Y. Peng, D. Zhao, K. Dai, W. Shi and K. Hirao, J. Phys. Chem. B, 2005, 109, 4947–4952 CrossRef CAS PubMed.
- J. Yang, Q. L. Dong, Z. T. Jiang and J. Zhang, Chin. Phys. B, 2010, 19, 127104 CrossRef.
- S. Bagwasi, Y. X. Niu, M. Nasir, B. Z. Tian and J. L. Zhang, Appl. Surf. Sci., 2013, 264, 139–147 CrossRef CAS PubMed.
- H. Y. Wang, Y. C. Yang, J. H. Wei, L. Le, Y. Liu, C. X. Pan, P. F. Fang, R. Xiong and J. Shi, React. Kinet., Mech. Catal., 2012, 106, 341–353 CrossRef CAS.
- A. Kongkanand and P. V. Kamat, ACS Nano, 2007, 1, 13–21 CrossRef CAS PubMed.
- D. Nassoko, Y. F. Li, H. Wang, J. L. Li, Y. Z. Li and Y. Yu, J. Alloys Compd., 2012, 540, 228–235 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |