Molecularly imprinted polymer nanospheres based on Mn-doped ZnS QDs via precipitation polymerization for room-temperature phosphorescence probing of 2,6-dichlorophenol†
Abstract
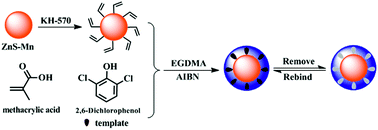
In this paper, novel molecularly imprinted polymers (MIPs) based on Mn doped ZnS quantum dots (QDs) with molecular recognition ability were successfully synthesized by precipitation polymerization using 2,6-dichlorophenol (2,6-DCP) as template, methacrylic acid (MAA) as the functional monomer and ethylene glycol dimethacrylate (EGDMA) as the cross-linker. The obtained materials (MIPs-ZnS:Mn QDs), which were composed of Mn doped ZnS QDs as phosphorescence signal and MIPs as molecular selective recognition sites, could sensitively and selectively recognize the template molecules by using the spectrofluorometer. After the experimental conditions were optimized, a linear relationship was obtained covering the range of 1.0–56 μmol L−1 with a correlation coefficient of 0.9994. The developed method was applicable to routine trace determination of 2,6-DCP in real examples. This study also provides a general strategy to fabricate MIPs-coated QDs with excellent performance and is desirable for chemical sensor application.

 Please wait while we load your content...
Please wait while we load your content...