Stable manganese carbonyl radicals as a rapid colorimetric thiol and hydrazine sensor†
Abstract
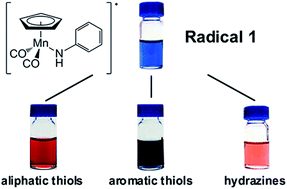
Blue cyclopentadienyl manganese dicarbonyl anilinyl radical complexes have been developed as a rapid and sensitive colorimetric thiol and hydrazine sensor with sensitivity limits approaching 3 ppm.

 Please wait while we load your content...
Please wait while we load your content...