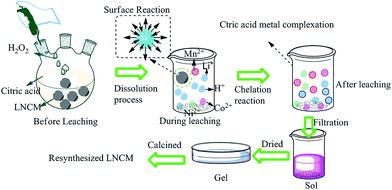
A new method for the synthesis of LiNi1/3Co1/3Mn1/3O2 from waste lithium ion batteries
Lu Yaoa,
Yong Fenga and
Guoxi Xi*ab
aSchool of Environment, Henan Normal University, Xinxiang 453007, PR China. E-mail: yaolu1020@126.com; yaolu001@163.com; Fax: +86 373 3326336; Tel: +86 373 3325796 Tel: +86 139 37399599
bSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, PR China
First published on 8th May 2015
Abstract
A new process for the synthesis of LiNi1/3Co1/3Mn1/3O2 from recycled valuable metals of waste lithium ion batteries (LIBs) is introduced herein. Citric acid was used as both a leaching and chelating agent. The overall process involves three steps: dissolution of spent lithium ion batteries, sol–gel formation and LiNi1/3Co1/3Mn1/3O2 powder formation. The concentrations of the metal ions, specifically lithium, nickel, manganese, and cobalt in the leachate were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES). The morphologies of the cathode material from waste LIBs before and after leaching, as well as those of the leaching residue and the final products, were observed by scanning electron microscopy (SEM). The structure of the final product was characterized by X-ray diffraction (XRD) analysis. With regards to electrochemical properties, the re-synthesized and fresh-synthesized samples delivered capacities of 147 and 150 mA h g−1, respectively, in the first cycle. The rate performances, high and low temperature discharge performances, and the electrochemical impedance spectra (EIS) are also discussed. This study suggests that the waste lithium ion batteries can be recycled to re-synthesize new cathode materials with good electrochemical performance.
1. Introduction
Lithium ion batteries (LIBs) are currently extensively used as electrochemical power sources in aerospace applications and electric vehicles because of their high power and energy density, high cell voltage, long storage life, low self-discharge rate, and wide operating temperature range.1–3 In recent years, increased production of electronic equipment and vehicles has led to more LIBs being manufactured, and hence a dramatic increase in the volume of depleted LIBs.Waste LIBs not only contain many flammable organic chemicals, but also many valuable metals, such as cobalt, nickel, aluminum, lithium, and manganese, and these metals are normally present at very high concentrations.4,5 Hence, waste LIBs represent an important secondary resource. Recycling of waste batteries has aroused widespread interest in recent years. Research has mainly focused on the recycling of waste LIBs with LiCoO2 as the cathode material, and many processes have been proposed for the recycling of cobalt from this source. The main processes for recycling waste LIBs are pyrometallurgy,6–8 hydrometallurgy,4,9–11 and biometallurgy.12–14 Pyrometallurgical processes are often accompanied by high gas emissions and have high energy consumption. The biometallurgical process is a very promising technology in terms of its high efficiency, low cost, and the modest apparatus needed, but the treatment period is too long and the bacteria required are difficult to incubate.5 Hence, the hydrometallurgy process has attracted the interest of many researchers and has become a focus of research work in recent years. Most of the hydrometallurgical methods involve acid leaching and the separation of metal ions. Sulfuric acid,10,15 nitric acid,16,17 and hydrochloric acid18 have high leaching efficiencies for cobalt and lithium. However, these strong acid solutions are liable to release SO3, NOx, and Cl2, respectively, and produce leachates of low pH, posing a threat to the environment and making them unfavorable for subsequent procedures. A number of organic acids are under development as potential leaching agent.4,9,15,16,19 However, except for the complicated separation of the metal ions, there has been little research on the subsequent treatment of the leachates.
Nowadays, the main industrial cathode materials are LiCoO2,16 LiMn2O4,20,21 LiNixCoyMn1−x−yO2(LNCM),22,23 and LiFePO4.24,25 Members of the LNCM family, with high discharge capacity, moderate voltage platform, and hence high energy density, have been considered as the most promising cathode candidates in recent years.22,26–29 Among them, LiNi1/3Co1/3Mn1/3O2 is an unique material because of its high specific capability.30 Its market share has increased year by year. However, research on the recycling of LNCM has been limited. The main research on recycling waste lithium ion batteries with LNCM as the cathode material has been focused on the recycling and separation of metal ions by inorganic acid leaching.31 Citric acid is inexpensive and non-hazardous, and could offer H+ and carboxyl groups for the leaching process and subsequent chelating process. In this study, we have recycled waste LIBs through an environmentally friendly process involving citric acid, which avoids complicated metal ion separation procedures. Moreover, a cathode material, LiNi1/3Co1/3Mn1/3O2, with high added value has been synthesized by a very simple method.
2. Materials and methods
2.1 Materials and reagents
The spent LIBs used in our study were kindly donated by Henan Huanyu Group (Xinxiang, China). All reagents used in this study were of analytical grade, and the solutions were prepared at the specified concentrations in distilled water.2.2 Experimental procedure
Immersion in N-methyl pyrrolidinone (NMP) and thermal treatment. The cathodes were immersed in NMP4,32 and heated to 80 °C for 1 h, and then transferred to an ultrasonic bath for 20 min. The ultrasonic treatment was intended to improve the efficiency and yield of separating the active cathode materials from the aluminum foils. The NMP extract was then filtered through a microfiltration membrane and the collected material was washed and dried at 100 °C. The recovered spent cathode material was calcined at 600 °C for 4 h in a muffle furnace to burn off impurities such as carbon and polyvinylidene fluoride (PVDF). The obtained powder was then ground for 30 min in a planetary ball mill for easier leaching.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at a total metal ion concentration of 1.0 mol L−1 by adding LiNO3, Mn(NO3)2 (50% solution), Ni(NO3)2·6H2O, and Co(NO3)2·6H2O, and the pH was adjusted to 8.0 by adding a suitable amount of aqueous ammonia. The transparent solution was heated at 80 °C in a water bath to obtain a transparent gel, which was then dried at 110 °C in an oven for 24 h. The dried gel was calcined at 350 °C for 2 h in the ambient atmosphere and then at 750 °C for 12 h in a muffle furnace, and the final product was designated as re-synthesized LiNi1/3Co1/3Mn1/3O2. For comparison, stoichiometric amounts of LiNO3, Ni(NO3)2·6H2O, Co(NO3)2·6H2O and Mn(NO3)2 (50% solution) were used as starting materials to synthesize LiNi1/3Co1/3Mn1/3O2 by the same method. This product was designated as fresh-synthesized LiNi1/3Co1/3Mn1/3O2.
1 at a total metal ion concentration of 1.0 mol L−1 by adding LiNO3, Mn(NO3)2 (50% solution), Ni(NO3)2·6H2O, and Co(NO3)2·6H2O, and the pH was adjusted to 8.0 by adding a suitable amount of aqueous ammonia. The transparent solution was heated at 80 °C in a water bath to obtain a transparent gel, which was then dried at 110 °C in an oven for 24 h. The dried gel was calcined at 350 °C for 2 h in the ambient atmosphere and then at 750 °C for 12 h in a muffle furnace, and the final product was designated as re-synthesized LiNi1/3Co1/3Mn1/3O2. For comparison, stoichiometric amounts of LiNO3, Ni(NO3)2·6H2O, Co(NO3)2·6H2O and Mn(NO3)2 (50% solution) were used as starting materials to synthesize LiNi1/3Co1/3Mn1/3O2 by the same method. This product was designated as fresh-synthesized LiNi1/3Co1/3Mn1/3O2.2.3 Analytical methods
The total metal ion concentration after acid leaching was determined by ICP-OES (model Optima 2100DV, USA). The microstructures of the cathode material before and after leaching were observed by SEM (Hitachi). Energy-dispersive X-ray spectroscopy (EDS) was carried out using an Oxford 7426 accessory as an SEM attachment, operated at an acceleration voltage of 20 kV. The phase of samples was determined by XRD analysis (D8 Advance, Bruker AXS, Germany) using Cu-Kα radiation. The electrochemical performances of the samples were studied by using CR2016-type coin cells. For the cathode assembly, LiNi1/3Co1/3Mn1/3O2 powder was thoroughly mixed with PVDF as a binder and two conducting media (Super-P and KS-6) at a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 in NMP to form an electrode slurry. This was pasted on an Al foil, the solvent was evaporated, and the prepared cathode sheets were dried in vacuo. Coin cells were then assembled in a glove box for electrochemical characterization. In the test cells, Li foil and a porous polypropylene film served as the counter electrode and the separator, respectively. The electrolyte was 1.0 mol L−1 LiPF6 in a mixture of ethylene carbonate, diethyl carbonate, and dimethyl carbonate with a weight ratio of 1
5 in NMP to form an electrode slurry. This was pasted on an Al foil, the solvent was evaporated, and the prepared cathode sheets were dried in vacuo. Coin cells were then assembled in a glove box for electrochemical characterization. In the test cells, Li foil and a porous polypropylene film served as the counter electrode and the separator, respectively. The electrolyte was 1.0 mol L−1 LiPF6 in a mixture of ethylene carbonate, diethyl carbonate, and dimethyl carbonate with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Charge–discharge experiments were performed on Land battery tests (Land CT2001A, Wuhan, China) between 2.75 and 4.25 V. The assembled cells were tested for 50 cycles at the current density of 1 C (140 mA g−1) to analyze cycling performance. Various rates (0.2, 1, 2, 3 and 5 C) were also tested to investigate the rate capability of the samples. Discharge tests were performed at −20 °C, 25 °C and 55 °C to analyze the performance of materials at high and low temperature. Electrochemical impedance spectra (EIS) were recorded using an electrochemical workstation (CHI 660D, Shanghai, Chenghua Company, China).
1. Charge–discharge experiments were performed on Land battery tests (Land CT2001A, Wuhan, China) between 2.75 and 4.25 V. The assembled cells were tested for 50 cycles at the current density of 1 C (140 mA g−1) to analyze cycling performance. Various rates (0.2, 1, 2, 3 and 5 C) were also tested to investigate the rate capability of the samples. Discharge tests were performed at −20 °C, 25 °C and 55 °C to analyze the performance of materials at high and low temperature. Electrochemical impedance spectra (EIS) were recorded using an electrochemical workstation (CHI 660D, Shanghai, Chenghua Company, China).
3. Results and discussion
3.1 Pre-treatment of cathodes from spent LIBs
SEM images of the active materials obtained from the cathodes before and after calcination are shown in Fig. 1a and b. Numerous small solid particles were seen between the active materials. These may have been of binder and conductor carbon added during manufacturing of the LIBs. It can be seen that most of these small particles vanished after calcination at 600 °C for 4 h, which we attribute to their combustion. An image of the residue obtained after leaching with aqua regia is shown in Fig. 1c, which reveals an amorphous structure. This may have been ash from combustion of the binder and conductor. Fig. 1a′–c′ show the corresponding EDS results. After calcination at 600 °C for 4 h, the weight ratio of carbon decreased significantly, which confirmed combustion of the binder and conductor. For the leaching residue, only three elements were detected, carbon at 70.87 wt%, oxygen at 19.37 wt% and a small amount of fluorine. No metal ions were detected in the residue. The elemental composition measured by EDS is listed in Table 1. | ||
| Fig. 1 SEM and EDS images of the waste LNCM: (a) the waste cathode material before calcination, (b) the cathode material after calcination and (c) the leaching residue. | ||
| Element | (a) Before calcined | (b) After calcined | (c) Residue | |||
|---|---|---|---|---|---|---|
| wt% | at% | wt% | at% | wt% | at% | |
| C | 29.24 | 47.75 | 3.81 | 9.21 | 70.87 | 77.39 |
| O | 30.20 | 37.03 | 25.81 | 46.79 | 19.37 | 15.86 |
| F | 1.91 | 1.98 | 8.36 | 12.77 | 9.78 | 6.75 |
| Ni | 17.90 | 5.98 | 29.37 | 14.51 | ||
| Co | 5.79 | 1.96 | 14.08 | 6.93 | ||
| Mn | 14.96 | 5.34 | 18.56 | 9.80 | ||
3.2 Leaching of waste LNCM
Citric acid is a common organic acid. Three carboxyl groups are contained in one C6H8O7 molecule. Theoretically, one mole of C6H8O7 could dissociate 3 moles of H+. However, not all of the H+ is released to the solution; the ionizable hydrogen dissociates in a stepwise manner. The dissociation of citric acid can be expressed as follows:| H3Cit = H2Cit− + H+ Ka1 = 7.4 × 10−4 |
| H2Cit− = HCit2− + H+ Ka2 = 1.7 × 10−5 |
| HCit2− = Cit3− + H+ Ka3 = 4.0 × 10−7 |
LiNi1/3Co1/3Mn1/3O2 has the α-NaFeO2 structure with space group R3m, which is also characteristic of layered LiCoO2. The valencies of nickel, cobalt, and manganese ions are +2, +3, and +4, respectively.33 In the crystal lattice, lithium ions occupy the 3a sites, the other metal ions, namely nickel, cobalt, and manganese, randomly occupy the 3b sites, and oxide anions occupy the 3c sites. Each transition metal ion is surrounded by six oxygen atoms, forming an MO6 octahedral structure, which is very stable. The reaction between LNCM and citric acid is a multiphase process. The chemical bonds between M (nickel, manganese, cobalt) and oxygen are extremely strong, which makes it difficult to leach with an organic acid. During the leaching process, one hydrogen ion can displace one lithium ion from LNCM. For L1−xNCM, during the charge–discharge process, different values of x give rise to different redox reactions. When x is between 0 and 1/3, the redox reaction of Ni2+/Ni3+ occurs. When x is between 1/3 and 2/3, the redox reaction of Ni3+/Ni4+ occurs. The main metal ions contained in the waste electrode are thus likely to be Li+, Ni2+, Ni3+, Ni4+, Co2+, Co3+, and Mn4+. Of these, Li+, Ni2+, and Co2+ may be readily leached by the acid, but Ni3+, Ni4+, Co3+, and Mn4+ would not be readily leached without a reducing agent. There are some reports that hydrogen peroxide (H2O2), can be oxidized by high-valent transition metal ions. For the recycling of LiCoO2, on adding H2O2 to citric acid, the leaching efficiencies of cobalt and lithium increased from 37% and 54% to 93% and 99%, respectively.19 In similar studies, on adding H2O2 in malic acid, aspartic acid and sulfuric acid, the leaching efficiencies of cobalt and lithium also increased dramatically.4,34,35 It is beneficial to deploy a reducing agent along with leaching acids. H. Zhou et al. found that waste LiNi1/3Co1/3Mn1/3O2 could be dissolve in 4 mol L−1 sulfuric acid with 30 wt% H2O2, and the leaching equation was as follows:36
| 6LiNi0.33Mn0.33Co0.33O2 + 9H2SO4 + H2O2 = 2MnSO4 + 2NiSO4 + 2CoSO4 + 3Li2SO4 + 2O2 + 10H2O |
H2O2 serves as a reductant in this system, Ni4+ serves as an oxidant, ϕθ Ni4+/Ni2+ = 1.678 V, ϕθ O2/H2O2 = 0.699 V, ΔEθ = ϕθ Ni4+/Ni2+ − ϕθ O2/H2O2 = 0.979 V, lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = nΔEθ/0.059 = 33, K = 1033. The stability constant K is sufficiently high and this redox reaction could proceed effectively. Similarly, Mn4+ and Co3+ could also be reduced by H2O2 in the citric acid solution.
K = nΔEθ/0.059 = 33, K = 1033. The stability constant K is sufficiently high and this redox reaction could proceed effectively. Similarly, Mn4+ and Co3+ could also be reduced by H2O2 in the citric acid solution.
So, in our experiment, we chose H2O2 to accelerate the leaching rates and efficiency. Thus the dissolution of nickel, manganese and cobalt ions follows a reduction-complex mechanism: M3+/4+}oxide + H+ + H2O2 → M2+}oxide → M2+}(aq.) → M(II)–citrate, (where M represents either nickel, cobalt or manganese).37 Such a heterogeneous reaction is expected to occur at the interface of solid (oxide lattice) and liquid. As the metal ions dissolves, the crystal lattice is disturbed. At the same time, the surrounding M ions may be leached into solution through complexation. All of the transition metal ions in this system, namely cobalt, nickel and manganese can be expected to form citrate complexes, because citrate is a strong complexing agent and was deployed in a stoichiometric amount (assuming 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal-to-ligand ratio).38 The leaching mechanism is shown schematically in Fig. 2.
2 metal-to-ligand ratio).38 The leaching mechanism is shown schematically in Fig. 2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L) on the leaching efficiency, experiments were conducted at S
L) on the leaching efficiency, experiments were conducted at S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L from 40 g L−1 to 120 g L−1, keeping the other parameters fixed as follows: leaching temperature 60 °C, citric acid concentration 1.0 mol L−1, H2O2 concentration 12 vol%, and a duration of 40 min. Fig. 3b shows that the leaching efficiency decreased dramatically with increasing S
L from 40 g L−1 to 120 g L−1, keeping the other parameters fixed as follows: leaching temperature 60 °C, citric acid concentration 1.0 mol L−1, H2O2 concentration 12 vol%, and a duration of 40 min. Fig. 3b shows that the leaching efficiency decreased dramatically with increasing S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L. As S
L. As S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L below 60 g L−1, more than 98% of the waste LIB cathode material was leached out. When the S
L below 60 g L−1, more than 98% of the waste LIB cathode material was leached out. When the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L exceeded 80 g L−1, however, the leaching efficiency decreased sharply. To achieve the best compromise between leaching efficiency and lower chemical consumption, leaching with a solid–liquid ratio of 80 g L−1 was determined to be optimal.
L exceeded 80 g L−1, however, the leaching efficiency decreased sharply. To achieve the best compromise between leaching efficiency and lower chemical consumption, leaching with a solid–liquid ratio of 80 g L−1 was determined to be optimal.| H2O2(l) → H2O(l) + 1/2O2(g) |
3.3 Synthesis and characterization of LiNi1/3Co1/3Mn1/3O2
An aliquot (15 mL) of the solution obtained after acid leaching and filtration was placed in a conical flask. The metal ion ratio was adjusted to 3.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at a total metal ion concentration of 1.0 mol L−1 by adding LiNO3, Ni(NO3)2·6H2O, Co(NO3)2·6H2O and Mn(NO3)2 (50% solution) as required. The solution was adjusted to pH 8.0 and stirred for 5 min. Another aliquot (15 mL) of the solution was used as a control. The two solutions were concentrated to dryness by freeze-drying. SEM images of the respective powders are shown in Fig. 4a and b. In Fig. 4b, it can be seen that the powder had a highly reticulated structure. This may be ascribed to the increased pH, which facilitated the second and third ionizations of citric acid, so that the system could supply more carboxyl groups to coordinate to the metal ions. The SEM image shows that a large reticular complex was formed when the solution was adjusted to pH 8.0 with aqueous ammonia, in accordance with the dissociation mechanism of citric acid.
1 at a total metal ion concentration of 1.0 mol L−1 by adding LiNO3, Ni(NO3)2·6H2O, Co(NO3)2·6H2O and Mn(NO3)2 (50% solution) as required. The solution was adjusted to pH 8.0 and stirred for 5 min. Another aliquot (15 mL) of the solution was used as a control. The two solutions were concentrated to dryness by freeze-drying. SEM images of the respective powders are shown in Fig. 4a and b. In Fig. 4b, it can be seen that the powder had a highly reticulated structure. This may be ascribed to the increased pH, which facilitated the second and third ionizations of citric acid, so that the system could supply more carboxyl groups to coordinate to the metal ions. The SEM image shows that a large reticular complex was formed when the solution was adjusted to pH 8.0 with aqueous ammonia, in accordance with the dissociation mechanism of citric acid.
 | ||
| Fig. 4 SEM images at different stage of the process: (a) the freeze-dried compound obtained from the leachate, (b) the freeze-dried compound obtained after adjusting the pH of the leachate to 8.0. | ||
 | ||
| Fig. 5 XRD patterns of the synthesized samples: (a) the re-synthesized sample, (b) the fresh-synthesized sample. | ||
SEM images of the re-synthesized and fresh-synthesized samples are shown in Fig. 6. No obvious differences between the two samples were apparent from SEM observation. Both the two powders consisted of well-crystallized relatively globular particles with smooth surfaces and uniform distributions. The particle diameters of the two samples were about 100–200 nm. In general, a small particle size can accelerate lithium-ion reactions between the electrode material and the electrolyte by reducing the diffusion path of lithium ions inside the particle, and the lithium ions can be easily inserted into and extracted from their sites or into defects. A small particle size of the cathode material is an important factor, as it gives a high surface area and greatly influences the electrochemical properties, such as rate capability and discharge capacity.
3.4 Electrochemical performance
In order to systematically evaluate the electrochemical performance of the samples, a series of electrochemical tests were performed. Charge–discharge profiles of the 1st, 2nd, 3rd, 10th, 20th, 50th cycles are shown in Fig. 7a. The re-synthesized and fresh-synthesized samples delivered capacities of 147 and 150 mA h g−1, respectively, in the first cycle. Fig. 7b shows the cycle properties of the re-synthesized and fresh-synthesized LiNi1/3Co1/3Mn1/3O2 samples between 2.75 and 4.25 V. No difference in the cycle performances of the two samples can be discerned. Both of the samples showed good cycling properties, and the capacity retention after 50 cycles reached 93%.The rate performances of the re-synthesized sample were incrementally tested from 0.2 C to 5 C (Fig. 7c). The discharge capacities of the re-synthesized sample were measured as 154.2, 147.0, 140.0, 136.7 and 108.6 mA h g−1 at 0.2, 1, 2, 3 and 5 C, respectively. At low charge–discharge rates, the discharge curves were flat and smooth, indicating that the electrolyte suitably surrounded the particles and electrolyte transfer could maintain the electrochemical process. At a relatively high charge–discharge rate (2 or 3 C), the electrolyte insufficiently surrounded the particles to support the electrochemical process, leading to a concentration polarization during electrolyte transfer. Thus, it produced a polarization effect on discharge curves. The small diameters and good dispersal of the particles facilitated electrolyte transfer, so a slow drop in discharge curves was observed. When the discharge rate reached 5 C, the electrochemical process was too fast. The electrolyte in pores was insufficient to support the electrochemical process, and therefore a serious polarization was generated, and the discharge curve showed an obvious drop.40 The rate capabilities of the re-synthesized and fresh-synthesized samples are compared in Fig. 7d. The average discharge capacities of the re-synthesized sample were 153.9, 146.4, 139.6, 113.5 and 106.9 mA h g−1 at 0.2, 1, 2, 3, and 5 C, respectively. When the discharge rate was returned to 0.2 C, about 90.1% (138.8 mA h g−1) of the discharge capacity was recovered. The freshly synthesized sample showed the same trends and there were no obvious differences between the two samples.
The discharge performances at high and low temperatures are shown in Fig. 8a. Discharge of the re-synthesized sample at −20, 25, and 55 °C led to discharge capacities of 105.8, 140.5, and 144.6 mA h g−1, respectively. It can clearly be seen that the discharge capacity increased with increasing temperature, especially below 25 °C. Concomitantly, the discharge voltage increased dramatically on increasing the temperature from −20 to 25 °C. On further increasing the temperature from 25 to 55 °C, there were no obvious increase in capacity or voltage.
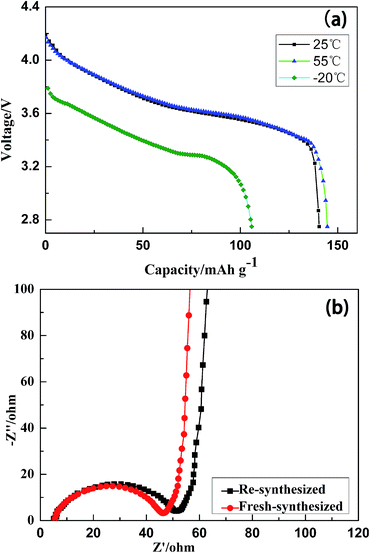 | ||
| Fig. 8 (a) Discharge profiles of the re-synthesized sample at −20, 25 and 55 °C at 1 C, (b) EIS plots of the re-synthesized and fresh-synthesized samples in fresh coin cells. | ||
EIS tests were conducted and the impedance plots are shown in Fig. 8b. Both samples exhibited typical Nyquist characteristic. The intercept in the high frequency region of the Z real axis corresponds to the ohmic resistance (Rs), the combined resistance of the electrolyte and the contacts of the cell. The semicircle at middle frequency is correlated with the Li+ charge transfer resistance at the interface.41 The inclined line in the lower frequency region represents the Warburg impedance (Zw), which is associated with the diffusion of Li+ in LiNi1/3Co1/3Mn1/3O2 particles.42
4. Conclusions
A simple and environmentally friendly recycling process has been developed for the recovery of lithium, cobalt, nickel, and manganese from waste LIBs. The process avoids complicated separation of the metal ions and produces minimal pollution and side products. The optimum leaching conditions were determined to be as follows: citric acid concentration 1.0 mol L−1, solid-to-liquid ratio 80 g L−1, hydrogen peroxide concentration 12 vol%, leaching temperature 60 °C, and reaction time 40 min. Under these conditions, more than 98% of the waste LIB cathode material could be leached. The leaching mechanism has been investigated from the viewpoint of the structure and dissociation mechanism of citric acid. The recovered and reconstituted cathode material showed high specific capacity, good rate and the cycling performances, almost matching those of the fresh-synthesized LiNi1/3Co1/3Mn1/3O2.Acknowledgements
This study was supported by the National Science Foundation of China (no. 51174083). It was also supported by the Specialized Research Fund for the Doctoral Program of Higher Education (no. 20114104110004).Notes and references
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243 CAS.
- J. Hassoun, B. Scrosati and Y. K. Sun, Energy Environ. Sci., 2011, 4, 3287 Search PubMed.
- D. Liu, W. Zhu, J. Trottier, C. Gagnon, F. Barray, A. Guerfi, A. Mauger, H. Groult, C. M. Julien, J. B. Goodenough and K. Zaghib, RSC Adv., 2014, 4, 154 RSC.
- L. Li, J. Ge, R. Chen, F. Wu, S. Chen and X. Zhang, Waste Manag., 2010, 30, 2615 CrossRef CAS PubMed.
- X. Zhang, Y. Xie, X. Lin, H. Li and H. Cao, J. Mater. Cycles Waste Manage., 2013, 15, 420 CrossRef CAS.
- J. F. Paulino, N. G. Busnardo and J. C. Afonso, J. Hazard. Mater., 2008, 150, 843 CrossRef CAS PubMed.
- L. Sun and K. Qiu, J. Hazard. Mater., 2011, 194, 378 CrossRef CAS PubMed.
- H. Nie, L. Xu, D. Song, J. Song, X. Shi, X. Wang and Z. Yuan, Green Chem., 2015, 17, 1276 RSC.
- L. Sun and K. Qiu, Waste Manag., 2012, 32, 1575 CrossRef CAS PubMed.
- M. K. Jha, A. Kumari, A. K. Jha, V. Kumar, J. Hait and B. D. Pandey, Waste Manag., 2013, 33, 1890 CrossRef CAS PubMed.
- S. Zhu, W. He, G. Li, X. Zhou, X. Zhang and J. Huang, Trans. Nonferrous Met. Soc. China, 2012, 22, 2274 CrossRef CAS.
- B. Xin, D. Zhang, X. Zhang, Y. Xia, F. Wu, S. Chen and L. Li, Bioresour. Technol., 2009, 100, 6163 CrossRef CAS PubMed.
- D. Mishra, D. Kim, D. E. Ralph, J. Ahn and Y. Rhee, Waste Manag., 2008, 28, 333 CrossRef CAS PubMed.
- G. Zeng, X. Deng, S. Luo, X. Luo and J. Zou, J. Hazard. Mater., 2012, 199–200, 164 CrossRef CAS PubMed.
- L. Ma, Z. Nie, X. Xi and X. Han, Hydrometallurgy, 2013, 136, 1 CrossRef CAS PubMed.
- L. Li, R. Chen, F. Sun, F. Wu and J. Liu, Hydrometallurgy, 2011, 108, 220 CrossRef CAS PubMed.
- L. Yang, Q. Yan, G. Xi, L. Niu, T. Lou, T. Wang and X. Wang, J. Mater. Sci., 2011, 46, 6106 CrossRef CAS.
- M. Joulié, R. Laucournet and E. Billy, J. Power Sources, 2014, 247, 551 CrossRef PubMed.
- L. Li, J. B. Dunn, X. Zhang, L. Gaines, R. Chen, F. Wu and K. Amine, J. Power Sources, 2013, 233, 180 CrossRef CAS PubMed.
- Y. Deng, L. Wan, Y. Xie, X. Qin and G. Chen, RSC Adv., 2014, 4, 23914 RSC.
- Q. Zhang, T. Peng, D. Zhan, X. Hu and G. Zhu, Mater. Chem. Phys., 2013, 138, 146 CrossRef CAS PubMed.
- M. Wang, G. Lei, J. Hu, K. Liu, S. Sang and H. Liu, RSC Adv., 2014, 4, 62615 RSC.
- L. Li, Z. Chen, Q. Zhang, M. Xu, X. Zhou, H. Zhu and K. Zhang, J. Mater. Chem. A, 2015, 3, 894 CAS.
- R. Tian, G. Liu, H. Liu, L. Zhang, X. Gu, Y. Guo, H. Wang, L. Sun and W. Chu, RSC Adv., 2015, 5, 1859 RSC.
- M. B. Sahana, S. Vasu, N. Sasikala, S. Anandan, H. Sepehri-Amin, C. Sudakar and R. Gopalan, RSC Adv., 2014, 4, 64429 RSC.
- J. Li, S. Xiong, Y. Liu, Z. Ju and Y. Qian, Nano Energy, 2013, 2, 1249 CrossRef CAS PubMed.
- S. Akimoto and I. Taniguchi, J. Power Sources, 2013, 242, 627 CrossRef CAS PubMed.
- A. Mahmoud, I. Saadoune, J. M. Amarilla and R. Hakkou, Electrochim. Acta, 2011, 56, 4081 CrossRef CAS PubMed.
- E. Shinova, R. Stoyanova, E. Zhecheva, G. Ortiz, P. Lavela and J. Tirado, Solid State Ionics, 2008, 179, 2198 CrossRef CAS PubMed.
- M. Sivakumar, A. Towata, K. Yasui, T. Tuziuti, T. Kozuka, Y. Iida, M. M. Maiorov, E. Blums, D. Bhattacharya, N. Sivakumar and M. Ashok, Ultrason. Sonochem., 2012, 19, 652 CrossRef CAS PubMed.
- G. Chen, X. Tang, Z. Wang, Q. Yi, L. Chen and Y. Xiao, Chin. J. Inorg. Chem., 2011, 27, 1987 CAS.
- C. Hu, J. Guo, J. Wen and Y. Peng, J. Mater. Sci. Technol., 2013, 29, 215 CAS.
- K. M. Shaju, G. V. Subba Rao and B. V. R. Chowdari, Electrochim. Acta, 2002, 48, 145 CrossRef CAS.
- L. Chen, X. Tang, Y. Zhang, L. Li, Z. Zeng and Y. Zhang, Hydrometallurgy, 2011, 108, 80 CrossRef CAS PubMed.
- D. A. Ferreira, L. M. Z. Prados, D. Majuste and M. B. Mansur, J. Power Sources, 2009, 187, 238 CrossRef CAS PubMed.
- H. Zou, E. Gratz, D. Apelian and Y. Wang, Green Chem., 2013, 15, 1183 RSC.
- G. P. Nayaka, J. Manjanna, K. V. Pai, R. Vadavi, S. J. Keny and V. S. Tripathi, Hydrometallurgy, 2015, 151, 73 CrossRef CAS PubMed.
- K. M. Nam, H. J. Kim, D. H. Kang, Y. S. Kim and S. W. Song, Green Chem., 2015, 17, 1127 RSC.
- B. J. Hwang, Y. W. Tsai, D. Carlier and G. Ceder, Chem. Mater., 2003, 15, 3676 CrossRef CAS.
- J. Song, L. Wang, Z. Ma, Z. Du, G. Shao, L. Kong and W. Gao, RSC Adv., 2015, 5, 1983 RSC.
- Y. Ding, J. Xie, G. Cao, T. Zhu, H. Yu and X. Zhao, Adv. Funct. Mater., 2011, 21, 348 CrossRef CAS PubMed.
- X. Yi, X. Wang, B. Ju, Q. Wei, X. Yang, G. Zou, H. Shu and L. Hu, J. Alloys Compd., 2014, 604, 50 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |