Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Theoretical design of tetra(arenediyl)bis(allyl) derivatives as model compounds for Cope rearrangement transition states†‡
L. Salvatella*
Instituto de Síntesis Química y Catálisis Homogénea – ISQCH, CSIC – Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza, Spain. E-mail: lsalvate@unizar.es
First published on 12th January 2015
Abstract
Several systems formed by two allyl fragments linked by four arenediyl tethers have been studied through DFT calculations. A delocalised bis(allyl) system, similar to Cope rearrangement transition states, is preferred for derivatives bearing 5-membered ring tethers, as a result of the large strain in the related localised geometry.
Despite the key role of transition states (TS's) in reaction mechanisms, very little data can be experimentally obtained for such structures. However, relevant structural information can instead be obtained from geometrically-constrained model compounds keeping the major features of the TS's for a number of reactions (such as proton1 and hydride2 transfer, SN2,3 1,2-alkyl shift—by using non-classical carbocations—,4 or phosphate transfer).5 As an application, drugs can be designed on the basis of their similitude to the convenient TS geometries.6
Some Cope rearrangement TS model compounds (showing two allyl fragments bound through two partial C⋯C bonds) have been designed by annelation of semibullvalene,7–10 barbaralane7 and bis-allyl11 frameworks through methylene,8,11 ethylene8,9 or oxydicarbonyl7 tethering. Although some of such model compounds have been characterised through UV-vis7,12 and IR10 spectroscopies, no X-ray diffraction data have been reported for such species up to now. In this work I report the theoretical results on a bis(allyl) system tethered through four arenediyl fragments as a new strategy to design delocalised compounds as models for typical Cope rearrangement TS's.
All calculations were carried out by using the Gaussian09 suit13 (see details in ESI‡). Geometries of all structures were obtained by using the restricted B3LYP (RB3LYP) method14 with the 6-311+G(d) basis set because of the excellent results of this technique on the Cope rearrangement activation barriers of hexa-1,5-diene (theoretical: 33.7 kcal mol−1; experimental: 33.3 kcal mol−1)15 and semibullvalene (theoretical: 3.8 kcal mol−1; experimental: 4.8 kcal mol−1).5 Unrestricted B3LYP (UB3LYP) calculations were not used for geometry optimisations because of the artifactual occurrence of an intermediate in the Cope rearrangement reaction of 2,5-dimethylidenehexanedinitrile at this level.16 Nevertheless, broken-symmetry UB3LYP energies were calculated on RB3LYP/6-311+G(d) geometries to take into account the possible diradicaloid character of the studied structures.17
Calculations on tetra(benzene-1,2-diyl)bis(allyl) (Ar = benzene-1,2-diyl; R1 = R2 = H, Scheme 1) showed the occurrence of energy minima for a Cs-symmetric localised structure as well as a C2v-symmetric delocalised framework, as a consequence of the destabilisation of the localised structure due to the large ring strain of two fused benzocyclobutane systems.18 Interestingly, the delocalised structure is slightly preferred (by 1.9 kcal mol−1 in Gibbs free energy).
With the aim of designing a more stable delocalised structure, the introduction of different substituents on the central carbon atom on one or two allyl systems was considered (Table 1). Interestingly, relative Gibbs free energies show that delocalised structures are destabilised by all kinds of substituents, similarly to experimental results on activation barriers for Cope rearrangement reactions.19 Delocalised structures are also destabilised by further tethers. For several systems, both localised and delocalised structures were identified as energy minima linked through a TS (see ESI‡), whereas delocalised structures showing relative Gibbs free energies larger than 1.0 kcal mol−1 were characterised as TS's.
| R1 | R2 | ΔGa | C–C distance | C/C distance | C⋯C distance |
|---|---|---|---|---|---|
| a Gibbs free energies calculated from UB3LYP/6-311+G(d)//RB3LYP/6-311+G(d) electronic energies and RB3LYP/6-311+G(d) thermal corrections.b Delocalised structure is a TS (showing one imaginary frequency). | |||||
| H | H | −1.9 | 1.788 | 2.447 | 2.294 |
| F | H | −1.3 | 1.765 | 2.453 | 2.287 |
| Me | H | −0.9 | 1.766 | 2.453 | 2.285 |
| NO2 | H | −0.5 | 1.765 | 2.438 | 2.276 |
| Cl | H | −0.5 | 1.761 | 2.446 | 2.277 |
| F | F | −0.5 | 1.744 | 2.453 | 2.288 |
| CN | H | −0.2 | 1.758 | 2.436 | 2.269 |
C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH |
H | −0.2 | 1.730 | 2.446 | 2.273 |
| Me | Me | 0.7 | 1.750 | 2.453 | 2.293 |
| NH2 | H | 1.2b | 1.737 | 2.481 | 2.292 |
| Cl | Cl | 1.9b | 1.761 | 2.446 | 2.269 |
| CN | CN | 2.8b | 1.735 | 2.436 | 2.247 |
C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH |
C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH |
3.3b | 1.730 | 2.449 | 2.256 |
–COCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO– CHCO– |
— | 0.7 | 1.745 | 2.433 | 2.252 |
| –NHCONH– | — | 1.4b | 1.747 | 2.418 | 2.237 |
| Naphthalene-1,8-diyl | — | 2.1b | 1.734 | 2.431 | 2.237 |
| –COOCO– | — | 2.2b | 1.733 | 2.410 | 2.217 |
| Benzene-1,2-diyl | — | 2.8b | 1.713 | 2.410 | 2.207 |
| Phenanthrene-4,5-diyl | — | 3.0b | 1.717 | 2.432 | 2.235 |
The localised structure for the non-substituted compound shows a very long Csp3–Csp3 bond (1.788 Å), significantly longer than that experimentally found for 9-tert-butyl-9,10-dewar anthracene (1.623 Å),18 also bearing two fused benzocyclobutane systems. Such an increased bond length can be attributed to an incipient σ-aromaticity. Accordingly, the Csp3–Csp3 bond shortening induced by all studied substituents on the localised framework can be attributed to a σ-aromaticity decrease due to the relative destabilisation of the delocalised structure.
All localised structures show similar non-bonded terminal allyl C/C distances (2.433–2.453 Å), excepting those bearing a further tether (2.410–2.453 Å) and the amino-derived framework (2.481 Å, due to the larger pyramidalisation of the terminal allyl carbon through NH2-vinyl conjugation). On the other hand, C⋯C bond lengths of delocalised energy minima (2.252–2.294 Å range) are similar to that calculated for the boat Cope rearrangement TS for hexa-1,5-diene (2.249 Å, at the same theoretical level).
As a second tactic to find a stable delocalised structure, replacement of benzene-1,2-diyl radicals by other tethers was considered (Table 2).
| Ar | ΔGa | 〈S2〉b | Singlet–triplet gapc | C–C distanced | C/C distanced | C⋯C distanced |
|---|---|---|---|---|---|---|
| a UB3LYP/6-311+G(d)//RB3LYP/6-311+G(d) calculations. Relative Gibbs free energies (at 25 °C, kcal mol−1).b Singlet-state wavefunction.c Relative electronic energies (kcal mol−1).d In Å.e Delocalised structure is a TS.f Localised structure could not be obtained. | ||||||
| Naphthalene-1,8-diyl | 6.5e | 0.000 | 61.2 | 1.740 | 2.650 | 2.333 |
| Benzene-1,2-diyl | −1.9 | 0.539 | 11.4 | 1.788 | 2.447 | 2.294 |
| Naphthalene-2,3-diyl | −3.3 | 0.613 | 9.6 | 1.819 | 2.454 | 2.326 |
| Thiophene-3,4-diyl | −14.0 | 0.988 | 1.6 | 1.876 | 2.526 | 2.441 |
| Pyrrole-3,4-diyl | — | 1.063 | 0.1 | —f | —f | 2.500 |
| Furan-3,4-diyl | — | 1.070 | 0.1 | —f | —f | 2.545 |
A significant dependence of the delocalised structure stability on the arenediyl tether was found. Thus, the localised structure is preferred for the naphthalene-1,8-diyl derivative, whereas the delocalised structure is predilected for naphthalene-2,3-diyl and thiophene-3,4-diyl derivatives (localised and delocalised structures being linked through a TS in all cases, see ESI‡). Finally, the delocalised structure was the only energy minimum for pyrrole-3,4-diyl and furan-3,4-diyl derivatives.
The preference for the delocalised structure in 5-membered cycle derivatives can be attributed to the high strain introduced by the cyclobutane-heterocycle fusion in localised structures. Thus, junction Csp2 bond angles (97.4° in the non-substituted benzene-1,2-diyl derivative) are significantly deformed relative to conventional sp2 bond angles (120°), and even more in comparison with typical angles in non-strained 5-membered rings (C3–C4–H angles: thiophene, 124.3°; furan, 126.1°; pyrrole, 127.1°).20
A significant dependence of the S2 expectation value was found for the UB3LYP wavefunction on the relative stability of the delocalised structure can be remarked: a pure singlet wavefunction for the napthalene-1,8-diyl derivative, a significant spin contamination (ca. 30% triplet contribution) for 6-membered ring tethered structures and a mixed spin-state (ca. 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 singlet–triplet) for 5-membered ring derived frameworks. Although the spin contamination in the singlet wavefunction is likely exaggerated by UB3LYP calculations (as inferred from singlet–triplet gap computations on acenes),21 a further stabilisation for all delocalised structures (excepting those lacking spin-contamination) was found by considering the “pure singlet” UB3LYP wavefunction (calculated from 〈S2〉 values and energies for singlet and triplet states through the Kraka formula, see ESI‡).22
50 singlet–triplet) for 5-membered ring derived frameworks. Although the spin contamination in the singlet wavefunction is likely exaggerated by UB3LYP calculations (as inferred from singlet–triplet gap computations on acenes),21 a further stabilisation for all delocalised structures (excepting those lacking spin-contamination) was found by considering the “pure singlet” UB3LYP wavefunction (calculated from 〈S2〉 values and energies for singlet and triplet states through the Kraka formula, see ESI‡).22
The decreasing trend of the singlet–triplet gap by increasing the structure strain (0.1 kcal mol−1 differences for pyrrole-3,4-diyl and furan-3,4-diyl derivatives) is analogous to that predicted for increasingly large acenes.23 Continuing this analogy, the predicted low singlet–triplet gap on higher acenes has not prevented the experimental synthesis of nonacene.24
Allyl–allyl C–C bond length in localised structures is dependent on the strain involved in the cycloalkane–(het)arene fusion. Thus, the relatively short bond for the naphthalene-1,8-diyl derivative can be attributed to the participation of a 5-membered (instead of 4-membered) ring. Instead, the ultralong C–C bond (1.876 Å) found for the thiophene derivative can be attributed to the high strain in 5-membered ring derivatives. Finally, delocalised structures could only be characterised as energy minima for pyrrole- and furan-derivatives.
Allyl–allyl terminal C/C distances of ca. 2.45 Å are found for benzene-1,2-diyl and naphthalene-2,3-diyl derivatives, whereas larger values are found for naphthalene-1,8-diyl (2.650 Å, due to the large separation between C1 and C8 atoms) and thiophene-3,4-diyl (2.526 Å, high ring strain) compounds.
A new family of compounds bearing ultralong C–C bonds25 can thus be formed due to the very long allyl–allyl distances found in delocalised structures (up to 2.545 Å), close to the C⋯C lengths found in Cope rearrangement TS's for some substituted hexadienes (hexa-1,5-diene-1,3,4,6-tetracarbonitrile, 2.467 Å;16 1,3,4,6-tetraphenylhexa-1,5-diene, 2.649 Å, according to RB3LYP/6-31G(d) calculations).26
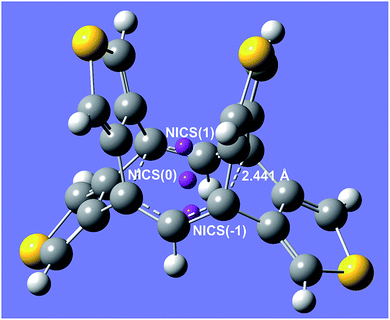
Aromaticity properties of σ-delocalised rings (illustrated for the thiophene-3,4-diyl derivative in Fig. 1) were confirmed by Nucleus-Independent Chemical Shifts (NICS)27 calculations on the UB3LYP wavefunction (see ESI‡) at the corresponding ring centre [NICS(0)] (typically in the −7.8/−4.9 ppm range, though more negative values are found in structures bearing a further tether, whereas 5-membered ring derivatives show −4.7/−3.4 ppm values, presumably due to the larger size of the aromatic ring),28 consistently with results on Cope rearrangement TS's.29 Similar qualitative conclusions can be drawn from NICS values for points placed at 1.0 Å distances from the ring centre towards the concave [NICS(1)] or convex [NICS(−1)] ring sides (though very low negative values are obtained for the former ones). On the other hand, the erratic behaviour found for the magnetic susceptibility variations on localised/delocalised rings (see ESI‡) may be attributed to the interference of the other aromatic rings.
Replacement of one or both allyl CH groups by nitrogen atoms in such bis(allyl) systems allows obtaining aza-Cope rearrangement TS models. Thus, a preference for the delocalised structure (by 17.1 kcal mol−1 in Gibbs free energy) has been found for the diaza-derivative bearing four furan-3,4-diyl tethers.
Conclusions
The theoretical study on tetra(arenediyl)bis(allyl) compounds indicate that several derivatives involving 5-membered rings can occur as energy minima bearing two allyl fragments bound by two equivalent partial C⋯C bonds. A new theoretical Cope rearrangement-based challenge for experimental chemists, analogously to those reported for σ-polyacenes30 or halogen-stabilised delocalised TS models,31 is thus outlined. I encourage experimental chemists to synthesise these new models for Cope rearrangement TS's. Although some difficulties might be found in such a synthesis, some tetra(arenediyl) derivatives of other frameworks [such as bis(ethylene)32 or bis(cyclobutane)]33 have already been characterised by X-ray diffraction analysis.Acknowledgements
I am indebted to the Instituto de Síntesis Química y Catálisis Homogénea (ISQCH) and the Instituto de Biocomputación y Física de Sistemas Complejos (BIFI) for allocation of computer time, as well as the Ministerio de Ciencia e Innovación of Spain (Project CTQ2011-28124), European Social Fund and the Gobierno de Aragón (Consolidated Group E11) for financial support.Notes and references
- A. F. Pozharskii, Russ. Chem. Rev., 1998, 67, 1 CrossRef PubMed.
- H. E. Katz, J. Am. Chem. Soc., 1985, 107, 1420 CrossRef CAS.
- J. C. Martin, Science, 1983, 221, 509 CAS.
- F. Scholz, D. Himmel, F. W. Heinemann, P. v. R. Schleyer, K. Meyer and I. Krossing, Science, 2013, 341, 62 CrossRef CAS PubMed.
- P. B. Rupert, A. P. Massey, S. T. Sigurdsson and A. R. Ferré-D'Amaré, Science, 2002, 298, 1421 CrossRef CAS PubMed.
- V. L. Schramm, ACS Chem. Biol., 2013, 8, 71 CrossRef CAS PubMed.
- A. C. Goren, D. A. Hrovat, M. Seefelder, H. Quast and W. T. Borden, J. Am. Chem. Soc., 2002, 124, 3469 CrossRef CAS PubMed.
- H. Jiao, R. Nagelkerke, H. A. Kurtz, R. V. Williams, W. T. Borden and P. v. R. Schleyer, J. Am. Chem. Soc., 1997, 119, 5921 CrossRef CAS.
- R. V. Williams and H. A. Kurtz, J. Org. Chem., 1988, 53, 3626 CrossRef CAS; P. v. R. Schleyer and H. Jiao, Pure Appl. Chem., 1996, 68, 209 Search PubMed.
- P. R. Griffiths, D. E. Pivonka and R. V. Williams, Chem.–Eur. J., 2011, 17, 9193 CrossRef CAS PubMed.
- D. J. Tantillo and R. Hoffmann, J. Org. Chem., 2002, 67, 1419 CrossRef CAS PubMed.
- H. Quast and M. Seefelder, Angew. Chem., Int. Ed., 1999, 38, 1064 CrossRef CAS; M. Seefelder and H. Quast, Angew. Chem., Int. Ed., 1999, 38, 1068 CrossRef.
- M. J. Frisch et al., Gaussian 09, Revision A.01, Gaussian Inc, Wallingford, CT, 2009 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS PubMed; C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef; P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623 CrossRef.
- H. Jiao and P. v. R. Schleyer, Angew. Chem., Int. Ed. Engl., 1995, 34, 334 CrossRef CAS.
- D. A. Hrovat, B. R. Beno, H. Lange, H.-Y. Yoo, K. N. Houk and W. T. Borden, J. Am. Chem. Soc., 1999, 121, 10529 CrossRef CAS.
- N. Graulich, WIREs Comput. Mol. Sci., 2011, 1, 172 CrossRef CAS.
- K. Angermund, K. H. Claus, R. Goddard and C. Krüger, Angew. Chem., Int. Ed. Engl., 1985, 24, 237 CrossRef.
- L. M. Jackman, E. Fernandes, M. Heubes and H. Quast, Eur. J. Org. Chem., 1998, 2209 CrossRef CAS.
- C. W. Bird and G. W. H. Cheeseman, Structure of Five-membered Rings with One Heteroatom, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon Press, Oxford, 1984, vol. 4, pp. 1–38 Search PubMed.
- S. Rayne and K. Forest, Comput. Theor. Chem., 2011, 976, 105 CrossRef CAS PubMed.
- J. Gräfenstein, A. M. Hjerpe, E. Kraka and D. Cremer, J. Phys. Chem. A, 2000, 104, 1748 CrossRef.
- B. Hajgató, M. Huzak and M. S. Deleuze, J. Phys. Chem. A, 2011, 115, 9282 CrossRef PubMed.
- C. Tönshoff and H. F. Bettinger, Angew. Chem., Int. Ed., 2010, 49, 4125 CrossRef PubMed.
- T. Suzuki, T. Takeda, H. Kawai and K. Fujiwara, Ultralong C–C bonds, in Strained Hydrocarbons, ed. H. Dodziuk and R. Hoffmann, Wiley-VCH, Chichester, 2009 Search PubMed.
- D. A. Hrovat, J. Chen, K. N. Houk and W. T. Borden, J. Am. Chem. Soc., 2000, 1222, 7456 CrossRef.
- P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. V. E. Hommes, J. Am. Chem. Soc., 1996, 118, 6317 CrossRef CAS.
- Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Chem. Rev., 2005, 105, 3842 CrossRef CAS PubMed.
- H. Jiao and P. v. R. Schleyer, J. Phys. Org. Chem., 1998, 11, 655 CrossRef CAS.
- D. J. Tantillo, R. Hoffmann, K. N. Houk, P. M. Warner, E. C. Brown and D. K. Henze, J. Am. Chem. Soc., 2004, 126, 4256 CrossRef CAS PubMed.
- S. C. Wang and D. J. Tantillo, J. Phys. Chem. A, 2007, 111, 7149 CrossRef CAS PubMed.
- R. L. Viavattene, F. D. Greene, L. D. Cheung, R. Majeste and L. M. Trefonas, J. Am. Chem. Soc., 1974, 96, 4342 CrossRef CAS.
- T. R. Battersby, P. Gantzel, K. K. Baldridge and J. S. Siegel, Tetrahedron Lett., 1995, 36, 845 CrossRef CAS.
Footnotes |
| † Dedicated to the memory of Prof. Paul von Ragué Schleyer. |
| ‡ Electronic supplementary information (ESI) available: Gaussian09 full reference, theoretical procedure as well as selected properties of all studied structures. See DOI: 10.1039/c4ra16381h |
| This journal is © The Royal Society of Chemistry 2015 |