Improved photocatalytic activity of g-C3N4 derived from cyanamide–urea solution†
Abstract
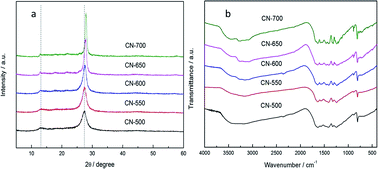
This paper describes the fabrication of g-C3N4 by the polymerization of cyanamide–urea solution at elevated temperatures. The textural properties and electronic band structure of the obtained g-C3N4 were investigated in detail. The photocatalytic activity for both oxidative and reductive reactions of the as-synthesized g-C3N4 was found to be enhanced as the polymerization temperature increase and the g-C3N4 obtained at 700 °C (CN-700) showed the best photocatalytic activity under visible-light (λ > 420 nm). Considering that the rather wide band gap (3.01 eV) of CN-700 disables the electron transition from the valence band to the conduction band by visible light (λ > 420 nm), it is believed the n–π* transition, which is alternatively proposed in this study, plays a key role in its photocatalytic activity. In light of this discovery, the variation of the electron-transition mechanism for g-C3N4 fabricated at different polymerization temperatures has been firstly investigated.

 Please wait while we load your content...
Please wait while we load your content...