Solvent-free heterogeneous catalysis for cyanosilylation in a modified sodalite-type Cu(ii)-MOF†
Abstract
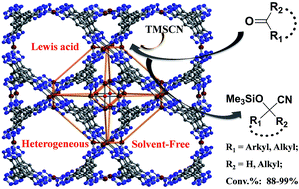
Solvothermal reaction of Cu(OAc)2 and H3BTT·2HCl generated a sodalite-type metal–organic framework [(Cu4O0.27Cl0.73)3(H0.5BTT)8(H2O)12]·3MeOH·9DMF (1) (BTT3− = 1,3,5-benzene tristetrazolate), which features a porous 3D (3,8)-connected framework constructed by square [Cu4(μ4-O/Cl)] units and triangular BTT ligands and can be dehydrated to form [(Cu4O0.27Cl0.73)3(H0.5BTT)8] (1′) with coordinatively unsaturated Cu2+ centers. Compared with related M–BTT MOFs, partial replacement of μ4-Cl with μ4-O at the square Cu4 cluster and the absence of irremovable guest [M(DMF)6]2+ cations in 1′ enhance the Lewis acidity of coordinatively unsaturated metal centers and the effective pore volume. Open Cu(II) sites with stronger Lewis acidity give rise to 1′ being an active, stable and reusable solvent-free heterogeneous catalyst for C–C bond-forming reaction by cyanosilylation of carbonyl compounds. The loading of 1 mol% of catalyst 1′ is as low as one eleventh of that used in related Mn–BTT but leads to as high as 96% conversion of benzaldehyde, indicating that the catalytic activity of M–BTT MOFs was significantly improved via post-modification. In addition, the larger pore volume makes 1′ selectively adsorb N2 and O2 gases with hysteresis loops over CO2 and H2 gases without hysteresis loops, which did not appear in related M–BTT MOFs.

 Please wait while we load your content...
Please wait while we load your content...