Urea formaldehyde (UF) microcapsules loaded with corrosion inhibitor for enhancing the anti-corrosive properties of acrylic-based multi-functional PU coatings†
Abstract
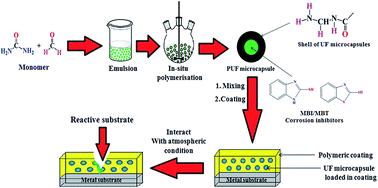
The present work focuses on enhancing the anti-corrosive performance of multi-functional polyurethane coatings by encapsulation of the commercially available inhibitors 2-mercaptobenzimidazole and 2-mercapatobenzothiozole, despite their reaction with diisocyanate. Initially, inhibitors were encapsulated by in situ polymerisation of urea and formaldehyde. Formation of microcapsules was confirmed by optical microscopy and FE-SEM. Thermal stability and particle size of microcapsules embedded with inhibitors were estimated by TGA and a particle size analyzer, respectively. Release rates of inhibitors were investigated by UV spectrophotometry. Anti-corrosive coatings were designed by dispersing encapsulated inhibitors in a multi-functional acrylic-based PU coating. The anti-corrosive study of coatings was investigated by the Tafel plot method, weight loss study, immersion study, and FE-SEM of corrosive panels. The impact of microcapsule concentration on the anticorrosive nature of coatings was also evaluated. Our study showed an increase in corrosion inhibition efficiency of PU coatings after incorporation of encapsulated corrosion inhibitors. Comparison of anti-corrosive performance of encapsulated inhibitors demonstrated that 2-MBT is superior to 2-MBI.

 Please wait while we load your content...
Please wait while we load your content...