Enhancing the electroluminescence performances of novel platinum(ii) polymetallayne-based phosphorescent polymers through employing functionalized IrIII phosphorescent units and facilitating triplet energy transfer†
Abstract
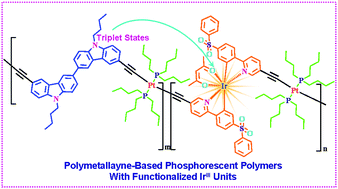
Novel orange phosphorescent polymers with platinum(II) polymetallayne-based backbones have been successfully developed through Sonogashira cross-coupling among bicarbazole moieties, functionalized IrIII phosphorescent blocks with electron injection/transporting (EI/ET) features, and trans-[PtCl2(PBu3)2]. Importantly, the very efficient energy-transfer process is observed from the triplet states of the polymetallayne-based backbone to the triplet metal-to-ligand charge transfer states (3MLCT) of the phosphorescent units in the polymer backbone, which will guarantee the high phosphorescent ability of these polymers. Benefiting from the weak conjugation-extending ability of the platinum(II) ions, the polymetallayne-based backbones show high triplet energy-level to effectively block the undesired reverse energy-transfer process. Furthermore, the EI/ET features of the functionalized IrIII phosphorescent units should balance the hole injection/transporting (HI/HT) of the bicarbazole moieties to improve the EL performances of these phosphorescent polymers. Benefiting from these merits, the phosphorescent polymers can furnish solution-processed phosphorescent OLEDs (PHOLEDs) with high EL efficiencies with current efficiency (ηL) of 9.17 cd A−1, external quantum efficiency (ηext) of 4.50% and power efficiency (ηP) of 4.04 lm W−1, representing very decent electroluminescent performances achieved by the orange phosphorescent polymers. This work herein might not only show the great potential of platinum(II) polymetallayne as the host segments in phosphorescent polymers, but also provide a new outlet to design and synthesise highly efficient phosphorescent copolymers.

 Please wait while we load your content...
Please wait while we load your content...