Low frequency ultrasound assisted sequential and co-precipitation syntheses of nanoporous RE (Gd and Sm) doped cerium oxide
Panneerselvam Sathishkumar*ab,
Ramalinga Viswanathan Mangalaraja*a,
Thangaraj Pandiyarajana,
M. A. Gracia-Pinillacd,
N. Escalonae,
C. Herrerae and
R. Garciae
aAdvanced Ceramics and Nanotechnology Laboratory, Department of Materials Engineering, Faculty of Engineering, University of Concepcion, Concepcion 407-0409, Chile. E-mail: sathish_panner2001@yahoo.com; mangal@udec.cl; Fax: +56 41 2203391; Tel: +56 41 2207389
bDepartment of Chemistry, Periyar Maniammai University, Vallam, Thanjavur 613403, Tamil Nadu, India
cUniversidad Autónoma de Nuevo León, Facultad de Ciencias Físico-Matemáticas, Av. Universidad, Cd. Universitaria, San Nicolás de los Garza, N.L., México
dUniversidad Autónoma de Nuevo León, Centro de Investigación e Innovación en Desarrollo de Ingeniería y Tecnología, Avenida Alianza 101 Sur PIIT Monterrey Apodaca, NL 66600, Mexico
eFaculty of Chemical Sciences, University of Concepcion, PO Box 160-C, Correo 3, Concepcion, Chile
First published on 12th February 2015
Abstract
Cerium oxide (CeO2), Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanostructures were synthesized using 42 kHz ultrasound assisted sequential and co-precipitation techniques. The nanoporous nature of the powders was revealed from the BET analysis which showed that the observed pore size distributions and the H2 hysteresis loops of the respective nanostructures confirmed the existence of nanopores in various sizes and shapes. The nanoporous nature of the synthesized powders were further confirmed by the high resolution transmission electron microscopy (HRTEM) and high angle annular dark field (HAADF) scanning transmission electron microscopy (STEM) analyses. The perceived shift in the characteristic F2g Raman active band centered at 464 cm−1 indicated the defects were increased during the sequential co-precipitation synthesis of Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanostructures. Moreover, the intensity of the F2g peaks can be ordered as CeO2 < Ce0.9Sm0.1O1.95 (CP)1 < Ce0.9Gd0.1O1.95 (SP)2 < Ce0.9Sm0.1O1.95 (SP)2 < Ce0.9Gd0.1O1.95 (CP)1 which indicates the increase in the concentration of the defects like oxygen vacancies enhances the efficiency of the solid oxide fuel cells (SOFCs) as electrolyte materials. The diffuse reflectance UV-vis solid state spectroscopic analysis demonstrated the narrowing optical band gap of CeO2 during the sequential and co-precipitation of the Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanostructures.
1. Introduction
The incessant usage of non-renewable energy resources leads to the initiation of two major threats to the economy of developing countries firstly, the scarcity and the cost of fossil fuels. The second and most important issue is the environmental pollution associated with the usage of fossil fuels which leads to unpredictable perilous effects throughout the world. To avoid the deleterious effects, fuel cells (FCs) are electrochemical devices which are non-polluting, have higher energy densities and efficiencies when compared to any other energy storage devices being produced.1,2 Among the various categories of FCs, solid oxide fuel cells (SOFCs) have become easier for the portable and stationary applications however the operating temperature needs to be reduced to improve the next generation of SOFCs. The operating principle of SOFCs can be simply explained as follows: the conversion of fuel into ions and electrons at the anode followed by the reaction of ions with the oxidants at the cathode.2,3 The electrolyte packed between the anode and the cathode generates the transport of the oxygen ions, which endorses the efficiency of the SOFCs.4 The enhanced physicochemical characteristics of the electrolytes certainly increase the efficiency of the SOFCs. The large scale preparation of the electrolytes through economically viable methodologies makes the electrolyte materials inexpensive.The nanomaterials synthesized by the ultrasound assisted technique exhibited various interesting and unconventional physicochemical characteristics. The utilization of low frequency or power ultrasound (20–100 kHz) has been significantly increased in the recent years for various applications.5–9 The extreme conditions (high temperature and pressure) produced during the ultrasound irradiation generate non-selective free radicals within the aqueous medium which accelerates the chemical reaction among the precursors and enhances the physicochemical characteristics of the subsequent nanomaterials. On the other hand, cerium dioxide (CeO2) is a highly refractive ceramic material which is utilized for the various applications of day-to-day life.10–15 The CeO2 and modified CeO2 electrolytes possess interesting features for SOFCs. The balanced behaviour of Ce3+/Ce4+ generates the properties which enable the significant contribution of CeO2 in SOFC applications. The doping of rare earth ions into the CeO2 significantly increases the oxygen vacancies and oxygen storage properties of the resulting electrolytes.16,17 Nevertheless, the loading of rare earth oxides (RE2O3) into CeO2 enhances the oxygen ion conductivity and the mechanical properties of the resultant nanomaterials.18,19
CeO2 and modified CeO2 nanomaterials prepared by using various procedures such as precipitation,20 hydrothermal,21 spray-pyrolysis,22 sol–gel23 and combustion24 were reported in the literature. The low frequency ultrasound assisted synthesis of CeO2 and modified CeO2 nanomaterials was expected to modify the physicochemical characteristics and to improve the thermal and mechanical properties of the resulting solid electrolytes. In the present work, CeO2, Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanostructures were synthesized by using 42 kHz ultrasound. The effect of sequential and co-precipitation on the structural, morphological and optical properties of the Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanostructures was investigated. The developed methodology can be extended for the preparation of various nanomaterials.
2. Experimental
2.1. Materials and methods
Nitrates of cerium, gadolinium and samarium, and sodium hydroxide were purchased from Sigma-Aldrich and used as starting materials for the syntheses of CeO2, Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanostructures without further purification. Unless otherwise specified, all reagents used were of analytical grade and the solutions were prepared using double distilled water. The crystallite sizes of the synthesized nanomaterials were calculated from the X-ray diffraction data (XRD, Philips PW1710 diffractometer, CuKα radiation, Holland) using the Scherrer equation. The surface morphologies and microstructures of the nanostructures were analyzed by transmission electron microscopy (HRTEM, FEI TITAN G2 80-300) operated at 300 keV. Diffuse reflectance UV-vis spectra of the nanostructures were recorded using a Shimadzu 2550 spectrophotometer equipped with an integrating sphere accessory employing BaSO4 as a reference material. Raman spectra were recorded using a Dilor LabRam-1B spectrometer, with a 633 nm line of He–Ne laser source with 5.5 mW power. The surface areas, pore volumes and pore diameters of the nanostructures were measured with the assistance of Flowsorb II 2300 of Micrometrics, Inc. The sonochemical reactions in this study were carried out using a commercially available sonicator (8890, Cole-Parmer, USA) producing 42 kHz ultrasonic waves.2.2. Preparation of CeO2, Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanostructures
The co-precipitation of Ce0.9Gd0.1O1.95 was carried out according to the procedure that we have reported earlier.25 For sequential precipitation, a slight modification in the synthesis procedure was carried out as follows: the appropriate quantity of the nitrate precursor of gadolinium and CeO2 was added to 200 mL of double distilled water under vigorous stirring for 15 min. 50 mL of 1 M NaOH was prepared separately. The sonicator was turned on and the time was taken as “time zero” for ultrasound irradiation at the same time as the NaOH was added drop-wise to the nitrate precursor under vigorous stirring. The ultrasound irradiation of the suspension was continued for 30 min. The solid solution was collected by subsequent filtration (0.45 μm nylon membrane filters). The solid solution was dried at 110 °C for 12 h followed by the calcination at 700 °C for 2 h. Similar procedure was adopted for the preparation of the Ce0.9Sm0.1O1.95, CeO2, Gd2O3 and Sm2O3 nanostructures. The stoichiometric concentration of CeO2 and nitrate precursors of gadolinium and samarium was used for the sequential precipitation of Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanocomposites.3. Results and discussion
The X-ray diffraction analysis of the nanomaterials synthesized using low frequency ultrasound is presented in Fig. 1. The formation of cubic structured Gd2O3 and Sm2O3 were identified according to the JCPDS numbers 74-1807 and 11-0608, respectively. The bare Sm2O3 demonstrated a poor crystalline nature when compared to the bare Gd2O3. The bare CeO2 exhibited a cubic fluorite crystal structure (JCPDS no. 34-0394) as evidenced by Fig. 1. The sequential and co-precipitation syntheses of Ce0.9Gd0.1O1.95, Ce0.9Sm0.1O1.95 revealed that the cubic fluorite crystal structure of CeO2 was not altered during the synthesis of the nanomaterials. The CeO2 diffraction pattern was dominant in the doped nanomaterials resulting from the sequentially and co-precipitated cerium (Ce4+) and dopant rare earth (Gd3+ and Sm3+) precursors. The observed strategy confirmed that the Gd3+ and Sm3+ entered into the crystal lattice of CeO2 which can be evidenced by the absence of the corresponding diffraction patterns of dopant rare earth oxides. However, the penetration of Gd3+ and Sm3+ in the sequentially precipitated nanostructures was likely to happen during the calcination and there was no core shell structure observed. Besides, further analysis is needed to confirm the morphology and crystal structure of the sequentially and co-precipitated Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanostructures. | ||
| Fig. 1 X-ray diffraction patterns of Gd2O3 (a), Sm2O3 (b), CeO2 (c), sequentially precipitated Ce0.9Gd0.1O1.95 (d), Ce0.9Sm0.1O1.95 (e) and co-precipitated Ce0.9Gd0.1O1.95 (f), Ce0.9Sm0.1O1.95 (g). | ||
The Raman spectra observed for the (co-precipitation and sequential precipitation) prepared nanostructures are shown in Fig. 2. They suggest that the vibrational changes occurred due to the introduction of rare earth oxides into the crystal structure of CeO2. The cubic fluorite crystal structures of CeO2, Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 can be identified from the strong and intense Raman active (F2g) bands at 455–470 cm−1. The oxygen vacancies in the crystal structure of CeO2 can be illustrated by the two less intensive bands which appear at the range of 250–290 and 605–625 cm−1.26 The CeO2 showed its characteristic F2g Raman active band centered at 464 cm−1, the sequentially and co-precipitated Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanostructures showed ∼± 2 nm shift in the F2g Raman active band for the cubic fluorite structure (inset of Fig. 2). The observed decrease in the intensity of the F2g band showed the increase in the concentration of defects like oxygen vacancies.29 The intensive Raman bands observed at 361 (Bg) and 345 cm−1 (Ag and Fg modes) suggested the cubic crystal structures of Gd2O3 and Sm2O3.27,28 The absence of the Raman active modes of Gd2O3 (361 cm−1), Sm2O3 (345 cm−1) and the shift observed at the F2g Raman active band for the cubic fluorite crystal structure (464 cm−1) clearly designated the incorporation of the rare earths into the crystal structure of CeO2. On the other hand, the intensity of the F2g peaks can be ordered as follows: CeO2 < Ce0.9Sm0.1O1.95 (CP)3 < Ce0.9Gd0.1O1.95 (SP)4 < Ce0.9Sm0.1O1.95 (SP)2 < Ce0.9Gd0.1O1.95 (CP)1.
 | ||
| Fig. 2 Raman spectra of various nanostructures (SP and CP denotes the sequential and co-precipitation syntheses). | ||
The HRTEM micrographs observed for the bare CeO2, Gd2O3 and Sm2O3 are presented in Fig. 3. The CeO2 exhibited nanoparticle morphology and the average grain size was ∼20 nm (Fig. 3a). The finger print lattice fringe distance was calculated as 0.31 nm (Fig. 3b) which corresponds to the (1 1 1) crystal plane of the cubic fluorite structure of CeO2. The micrographs of Gd2O3 and Sm2O3 revealed nanorod morphologies of several nanometers in length and thickness along with approximately 15% nanoparticle morphology (Fig. 3c and e). The finger print lattice fringe distances were calculated as 0.31 nm and 0.32 for the corresponding (2 2 2) crystal planes of Gd2O3 (Fig. 3d) and Sm2O3 (Fig. 3f), respectively. The HRTEM micrographs of the sequentially and co-precipitated Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanostructures demonstrated the formation of clear internal crystal lattice structures (Fig. 4 and 5). This confirmed that high crystallinity was achieved during the synthesis of the nanomaterials. The HRTEM micrographs (Fig. 4) of the sequentially (Fig. 4 a–c) and co-precipitated (Fig. 4 d–f) Ce0.9Gd0.1O1.95 nanostructures demonstrated that the significant number of bare Gd2O3 nanorods (Fig. 3c) were transformed to the nanoparticle morphology.
 | ||
| Fig. 4 HRTEM micrographs of sequentially precipitated Ce0.9Gd0.1O1.95 (a–c) and co-precipitated Ce0.9Gd0.1O1.95 (d–f). | ||
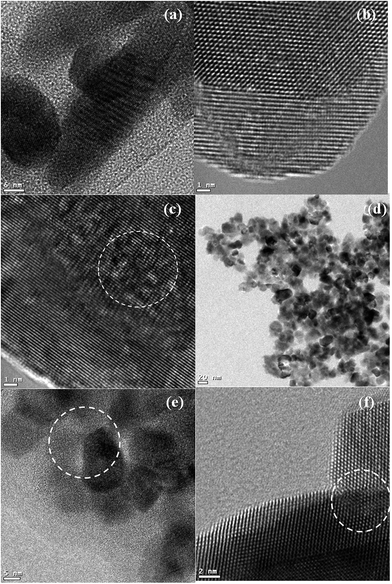 | ||
| Fig. 5 HRTEM micrographs of sequentially precipitated Ce0.9Sm0.1O1.95 (a–c) and co-precipitated Ce0.9Sm0.1O1.95 (d–f). | ||
The HRTEM micrographs (Fig. 5) of the sequentially precipitated (Fig. 5 a–c) Ce0.9Sm0.1O1.95 nanostructures showed 25% nanorod morphology whereas the co-precipitated (Fig. 5 d–f) Ce0.9Sm0.1O1.95 nanostructures showed only 5% nanorod morphology. However, the ratio of nanorods versus nanoparticles had significantly decreased compared to the bare Sm2O3 (Fig. 3e). A similar kind of surface distribution of samarium was detected on the surface of CeO2 when compared with the Ce0.9Gd0.1O1.95. This confirmed that the 42 kHz low frequency ultrasound was adequate to stimulate the initiation of Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanostructures from the precursors. The grain size achieved for the Ce0.9Gd0.1O1.95 (Fig. 4a and d) and Ce0.9Sm0.1O1.95 (Fig. 5a and d) nanopowders was calculated to be ∼10 nm from the HRTEM analysis. The calculated grain size from the HRTEM analysis was in good agreement with the grain size calculated from the XRD patterns using the Debye–Scherrer equation. The observed decrease in the grain size suggested an increase in the defects of individual nanomaterials.30
The formation of nanoporous structures is shown in Fig. 4b, c and f, 5c, e and f (circled). The HRTEM micrographs additionally demonstrated the formation of Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 when the precursors underwent the ultrasonic irradiation. The sequentially precipitated nanostructures exhibited a greater number of nanopores when compared to the co-precipitated materials. The formation of the nanoporous structure was not observed for the bare Gd2O3 and Sm2O3 under the same laboratory conditions. The nanoporous structure of CeO2 was noticed from Fig. 3a, however the HRTEM analysis evidently suggested that the number of nanopores in CeO2 was found to increase during the synthesis of the rare earth doped CeO2.
The high angle annular dark field scanning transmission electron microscopy (HAADF-STEM) analysis of the sonochemically synthesized Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 revealed the formation of nanopores which can be easily identified as the black blotches in the micrographs (Fig. 6a and b & Fig. 7a and b). The diameters of the nanopores were measured as ∼1 to 2 nm during the HAADF-STEM analysis. The sequentially precipitated Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 exhibited a large number of nanopores when compared to the co-precipitated nanostructures. The HAADF emission by analysis confirmed the formation of Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 and no other phases belonging to impurities were detected. The EDX analysis of the corresponding nanomaterials is presented in Fig. 6c and d & Fig. 7c and d. The elemental analysis indicated the stoichiometric ratio of the nanomaterials.
 | ||
| Fig. 6 HAADF-STEM micrographs of sequentially precipitated Ce0.9Gd0.1O1.95 (a), Ce0.9Sm0.1O1.95 (b) and the corresponding EDX images (c and d). | ||
 | ||
| Fig. 7 HAADF-STEM micrographs of co-precipitated Ce0.9Gd0.1O1.95 (a), Ce0.9Sm0.1O1.95 (b) and the corresponding EDX images (c and d). | ||
Brunar, Emmett and Teller (BET) analysis was performed for the sonochemically synthesized nanostructures to understand the various structural and textural properties of CeO2, sequentially and co-precipitated Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95. Fig. 8 shows the type IV adsorption–desorption profiles measured for all the sonochemically synthesized nanopowders. The CeO2, Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 adsorption–desorption profiles demonstrated the narrow H2 type hysteresis loop which was not observed for Gd2O3 and Sm2O3. The observed H2 type hysteresis loops revealed the complex pore structures which can also be evidenced by the HRTEM and HAADF-STEM analyses. The surface areas and the various parameters associated with the pores are presented in Table 1 which illustrates the decrease in the surface area of the sequentially precipitated Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 compared to the bare CeO2. Besides, Barrett–Joyner–Halenda (BJH) analysis (Fig. 9) showed that the pore volume distribution attained for the bare CeO2 was not significantly altered during the sequential precipitation. However, the H2 hysteresis observed for co-precipitated rare earth doped ceria was broader (Fig. 8) than the bare CeO2. Also, the surface areas and pore diameters of the co-precipitated Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 were modified compared to the bare CeO2 (Table 1 and Fig. 9). Considerable changes occurred during co-precipitation indicated by the formation of different sized and shaped nanopores31 under the low frequency ultrasound assisted process. Moreover, Table 1 clarifies the various nanoporous properties of the synthesized nanostructures.
 | ||
| Fig. 8 BET analysis of various nanostructures (SP and CP denotes the sequential and co-precipitation). | ||
| S. no. | Nanopowder | BET surface area (m2 g−1) | Total pore volume (cm3 g−1) | Micropore volume (cm3 g−1) | Mesopore volume (cm3 g−1) | Pore diameter (nm) |
|---|---|---|---|---|---|---|
| a Co-precipitation.b Sequential precipitation. | ||||||
| 1. | CeO2 | 41 | 0.11 | 0.02 | 0.09 | 11.7 |
| 2. | Ce0.9Gd0.1O1.95 (CP)a | 48 | 0.10 | 0.02 | 0.08 | 8.3 |
| 3. | Ce0.9Sm0.1O1.95 (CP)a | 38 | 0.09 | 0.02 | 0.08 | 9.7 |
| 4. | Gd2O3 | 23 | 0.04 | 0.01 | 0.03 | 7.1 |
| 5. | Sm2O3 | 30 | 0.05 | 0.01 | 0.04 | 7.1 |
| 6. | Ce0.9Gd0.1O1.95 (SP)b | 36 | 0.10 | 0.02 | 0.08 | 11.9 |
| 7. | Ce0.9Sm0.1O1.95 (SP)b | 37 | 0.10 | 0.02 | 0.08 | 11.8 |
The diffuse reflectance (DR)-UV-vis spectral analysis of the synthesized nanostructures is presented in Fig. 10. The bare Gd2O3 and Sm2O3 showed their absorption maxima in the ultraviolet region and the absorption of bare CeO2 can be indexed in the visible light region. The characteristic visible light absorption of the bare CeO2 considerably demonstrated that the number of defects (fluorite crystal structure) was increased during the ultrasound irradiations which led to a shift in the characteristic absorption when compared to the commercial CeO2.32 The absence of the characteristic absorption of Gd2O3 and Sm2O3 in the DR-UV-vis spectra also supported the formation of Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 in addition to the Raman and HRTEM analyses. The absorption band edge was blue shifted for the sequentially precipitated Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 whereas it was red shifted for the co-precipitated Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 when compared to the same band of the bare CeO2 (Fig. 10). The Tauc plot derived from the Kubelka–Munk function is shown in Fig. 11. The optical band gap of the bare CeO2 was 2.228 eV which was significantly lower than the band gap reported for commercial CeO2.32 The optical band gaps of the sequentially precipitated Ce0.9Gd0.1O1.95 (2.321 eV) and Ce0.9Sm0.1O1.95 (2.410 eV) were higher than the bare CeO2 (2.228 eV). Besides, the co-precipitated Ce0.9Gd0.1O1.95 (2.214 eV) and Ce0.9Sm0.1O1.95 (2.212 eV) showed narrower optical band gaps than CeO2. The respective properties of Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95, when compared to the optical band gap of bare Gd2O3 (5.05 eV), Sm2O3 (4.5 eV) and CeO2, indicated that the rare earth oxides acted as band gap modifiers during the ultrasound assisted sequential and co-precipitation.
 | ||
| Fig. 10 Diffuse reflectance (DR)-UV-vis spectra of various nanostructures (SP and CP denotes the sequential and co-precipitation). | ||
 | ||
| Fig. 11 Tauc plot of the synthesized nanostructures calculated from the DR-UV-vis spectrum using the Kubelka and Munk function (SP and CP denotes the sequential and co-precipitation). | ||
4. Conclusion
The CeO2, Gd2O3, Sm2O3, Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 nanostructures were successfully synthesized using 42 kHz ultrasound assisted sequential and co-precipitation techniques. The nanorod morphologies of the Gd2O3 and Sm2O3 were transformed into nanoparticle morphologies during the preparation of Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95. The HRTEM analysis significantly indicated the decrease in the grain sizes of the sequential and co-precipitated Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 compared to the bare CeO2, Gd2O3 and Sm2O3. In accordance with the grain size, BET and BJH analyses illustrated that the surface areas, nanoporous textures and pore diameters of the rare earth doped nanoceria were modified compared to the bare and commercially available CeO2. Moreover, the observed shift shown in the Raman spectra and the band gap narrowing during the diffuse reflectance UV-vis investigation illustrated that the defects (oxygen vacancies) increased in the Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 compared to the bare CeO2, Gd2O3 and Sm2O3. The increased number of nanopores was identified from the HAADF-STEM analysis and the BET analysis demonstrated that the pore diameter of the sequentially precipitated rare earth doped nanoceria was not significantly modified compared to the bare CeO2 and co-precipitated rare earth doped nanoceria. Therefore, the ultrasound assisted synthesis of Ce0.9Gd0.1O1.95 and Ce0.9Sm0.1O1.95 will make a significant contribution to improve the ion conducting properties which enhance the efficiency of solid oxide fuel cells.Acknowledgements
The authors would like to thank FONDECYT no. 1130916 Government of Chile, Santiago, for financial assistance.References
- Fuel cell technology: reaching towards commercialization, ed. N. Sammes, Springer, London, 2006 Search PubMed.
- A. Hermanna, T. Chaudhuria and P. Spagnol, Bipolar plates for PEM fuel cells: A review, Int. J. Hydrogen Energy, 2005, 30, 1297–1302 CrossRef PubMed.
- S. Srinivasan, Fuel cells: from Fundamentals to applications, New York, Springer, 2006 Search PubMed.
- S. P. Jiang, Development of lanthanum strontium manganite perovskite cathode materials of solid oxide fuel cells: a review, J. Mater. Sci., 2008, 43, 6799–6833 CrossRef CAS PubMed.
- S. Pilli, P. Bhunia, S. Yan, R. J. LeBlanc, R. D. Tyagi and R. Y. Surampalli, Ultrasonic pretreatment of sludge: A review, Ultrason. Sonochem., 2011, 18, 1–18 CrossRef CAS PubMed.
- H. Okkay, M. Bayramoglu and M. F. Oksuzomer, Ce0.8Sm0.2O1.9 synthesis for solid oxide fuel cell electrolyte by ultrasound assisted co-precipitation method, Ultrason. Sonochem., 2013, 20, 978–983 CrossRef CAS PubMed.
- S. Cho, J. Kim, J. Chun and J. Kim, Ultrasonic formation of nanobubbles and their zeta-potentials in aqueous electrolyte and surfactant solutions, Colloids Surf., A, 2005, 269, 28–34 CrossRef CAS PubMed.
- Y. Wang, D. Zhao, W. Ma, C. Chen and J. Zhao, Enhanced sonocatalytic degradation of azo dyes by Au/TiO2, Environ. Sci. Technol., 2008, 42, 6173–6178 CrossRef CAS.
- P. Sathishkumar, R. V. Mangalaraja, H. D. Mansilla, M. A. Gracia-Pinilla and S. Anandan, Sonophotocatalytic (42 kHz) degradation of Simazine in the presence of Au/TiO2 nanocatalysts, Appl. Catal., B, 2014, 160–161, 692–700 CrossRef CAS PubMed.
- P. Trogadas, J. Parrondo and V. Ramani, Platinum supported on CeO2 effectively scavenges free radicals within the electrolyte of an operating fuel cell, Chem. Commun., 2011, 47, 11549–11551 RSC.
- P. Jasinski, T. Suzuki and H. U. Anderson, Nanocrystalline undoped ceria oxygen sensor, Sens. Actuators, B, 2003, 95, 73–77 CrossRef CAS.
- T. Mori and J. Drennan, Influence of microstructure on oxide ionic conductivity in doped CeO2 electrolytes, J. Electroceram., 2012, 17, 749–757 CrossRef PubMed.
- X. Lu, T. Zhai, H. Cui, H. Shi, S. Xie, Y. Huang, C. Liang and Y. Tong, Redox cycles promoting photocatalytic hydrogen evolution of CeO2nanorods, J. Mater. Chem., 2011, 21, 5569–5572 RSC.
- S. Yabea and T. Satob, Cerium oxide for sunscreen cosmetics, J. Solid State Chem., 2003, 171, 7–11 CrossRef.
- J. F. de Lima, R. F. Martins, C. R. Neri and O. A. Serra, ZnO:CeO2-based nanopowders with low catalytic activity as UV absorbers, Appl. Surf. Sci., 2009, 255, 9006–9009 CrossRef PubMed.
- B. Matović, J. Dukić, A. Devečerski, S. Bošković, M. Ninić and Z. Dohčević-Mitrović, Crystal structure analysis of Nd-doped ceria solid solutions, Sci. Sintering, 2008, 40, 63–68 CrossRef.
- B. Choudhury and A. Choudhury, Lattice distortion and corresponding changes in optical properties of CeO2 nanoparticles on Nd doping, Curr. Appl. Phys., 2013, 13, 217–223 CrossRef PubMed.
- T. S. Zhang, J. Ma, L. B. Kong, P. Hing and J. A. Kilner, Preparation and mechanical properties of dense Ce0.8Gd0.2O2−δ, ceramics, Solid State Ionics, 2004, 167, 191–196 CrossRef CAS PubMed.
- A. Akbari-Fakhrabadi, R. V. Mangalaraja, F. A. Sanhueza, R. E. Avila, S. Ananthakumar and S. H. Chan, Nanostructured Gd–CeO2 electrolyte for solid oxide fuel cell by aqueous tape casting, J. Power Sources, 2012, 218, 307–312 CrossRef CAS PubMed.
- A. I. Y. Tok, L. H. Luo and F. Y. C. Boey, Carbonate co-precipitationof Gd2O3-doped CeO2 solid solution nano-particles, Mater. Sci. Eng., A, 2004, 383, 229–234 CrossRef PubMed.
- F. Zhang, S. P. Yang, H. M. Chen and X. B. Yu, Preparation of discrete nano size ceria powder, Ceram. Int., 2004, 30, 997–1002 CrossRef CAS PubMed.
- C. Goulart and E. Djurado, Synthesis and sintering of Gd-doped CeO2 nanopowders prepared by ultrasonic spray pyrolysis, J. Eur. Ceram. Soc., 2013, 33, 769–778 CrossRef CAS PubMed.
- G. S. Wua, T. Xiea, X. Y. Yuana, B. C. Cheng and L. D. Zhang, An improved sol–gel template synthetic route to large-scale CeO2 nanowires, Mater. Res. Bull., 2004, 39, 1023–1028 CrossRef PubMed.
- L. D. Jadhava, M. G. Chourashiya, K. M. Subhedar, A. K. Tyagi and J. Y. Patil, Synthesis of nanocrystalline Gd doped ceria by combustion technique, J. Alloys Compd., 2009, 470, 383–386 CrossRef PubMed.
- P. Sathishkumar, R. V. Mangalaraja, O. Rozas, H. D. Mansilla, M. A. Gracia-Pinilla and S. Anandan, Low frequency ultrasound (42 kHz) assisted degradation of Acid Blue 113 in the presence of visible light driven rare earth nanoclusters loaded TiO2 nanophotocatalysts, Ultrason. Sonochem., 2014, 21, 1675–1681 CrossRef CAS PubMed.
- R. Kydd, W. Y. Teoh, K. Wong, Y. Wang, J. Scott, Q. H. Zeng, A. B. Yu, J. Zou and R. Amal, Flame-synthesized ceria-supported copper dimers for preferential oxidation of CO, Adv. Funct. Mater., 2009, 19, 369–377 CrossRef CAS.
- C. Le Luyer, A. Garcia-Murillo, E. Bernstein and J. Mugnier, Waveguide Raman spectroscopy of sol–gel Gd2O3 thin films, J. Raman Spectrosc., 2003, 34, 234–239 CrossRef CAS.
- S. Jiang, J. Liu, C. Lin, X. Li and Y. Li, High-pressure X-ray diffraction and Raman spectroscopy of phase transitions in Sm2O3, J. Appl. Phys., 2013, 113, 113502–113506 CrossRef PubMed.
- X. Yao, C. Tang, Z. Ji, Y. Dai, Y. Cao, F. Gao, L. Dong and Y. Chen, Investigation of the physicochemical properties and catalytic activities of Ce0.67M0.33O2 (M = Zr4+, Ti4+, Sn4+) solid solutions for NO removal by CO, Catal. Sci. Technol., 2013, 3, 688–698 CAS.
- L. Ilieva, G. Pantaleo, I. Ivanov, A. M. Venezia and D. Andreeva, Gold catalysts supported on CeO2 and CeO2–Al2O3 for NOx reduction by CO, Appl. Catal., B, 2006, 65, 101–109 CrossRef CAS PubMed.
- A. Grosman and C. Ortega, Capillary condensation in porous materials. Hysteresis and interaction mechanism without pore blocking/percolation process, Langmuir, 2008, 24, 3977–3986 CrossRef CAS PubMed.
- S. A. Ansari, M. M. Khan, M. O. Ansari, S. Kalathil, J. Lee and M. H. Cho, Band gap engineering of CeO2 nanostructure using an electrochemically active biofilm for visible light applications, RSC Adv., 2014, 4, 16782–16791 RSC.
| This journal is © The Royal Society of Chemistry 2015 |







