Comparative evaluation of the blends of gas-to-liquid (GTL) fuels and biodiesels with diesel at high idling conditions: an in-depth analysis on engine performance and environment pollutants
S. Hossain*,
H. H. Masjuki*,
M. Varman,
M. A. Kalam and
S. M. Ashrafur Rahman
Centre for Energy Sciences, Department of Mechanical Engineering, Faculty of Engineering, University of Malaya, 50603 Kuala Lumpur, Malaysia. E-mail: s.hossainme@gmail.com; masjuki@um.edu.my; Fax: +603 79675317
First published on 9th January 2015
Abstract
This study focuses on the physicochemical fuel characteristics and engine performance-emission features of three prospective alternative transportation fuels: Alexandrian laurel biodiesel (ALBD), jatropha biodiesel (JBD) and GTL fuel at high idling conditions. The blends of GTL fuel (G10, G20), JBD (J10, J20) and ALBD (AL10, AL20) with diesel had been investigated in a multi-cylinder diesel engine at different load-speed conditions. Analysis of the fuel properties showed a linear variation of the major fuel properties with an increase of alternative fuel quantity in the blends. Engine performance test results revealed an average decrease of brake specific fuel consumption (BSFC) (ca. 8.65–12.26%) and brake specific energy consumption (BSEC) (ca. 8.27–11.51%), but a higher brake thermal efficiency (BTE) (ca. 8.56–12.58%) by GTL blends, whereas, the biodiesel blends showed higher BSFC (ca. 5.01–12.18%) and BSEC (ca. 3.41–9.67%) and lower BTE (ca. 3.68–9.93%), respectively, than those of diesel. Referring to the emission analysis, the results revealed that GTL blends showed a slight reduction in NOx (ca. 3.89–6.85%), but a significant reduction in CO (ca. 48.25–51.38%), HC (ca. 44.81–51.43%) and smoke (ca. 15.21–18.78%), respectively, when compared to diesel. The biodiesel blends demonstrated reduced CO (on average ca. 29.12–33.71%), HC (ca. 29.67–35.46%) and smoke (ca. 2.49–6.87%), but increased NOx (on average ca. 2.83–9.81%), respectively, than those of diesel.
1. Introduction
1.1. Background
Worldwide petroleum upheaval and environmental distress have heightened the urge for research and development of alternative fuels in the transportation sector. Biodiesel and gas-to-liquid (GTL) fuel can be considered as prospective future transportation fuels. Biodiesel is defined as the mono-alkyl esters of fatty acids, which can be extracted from vegetable oils, animal fats and alcohol. It has special features such as being renewable, biodegradable and free from toxicity; contains a higher cetane number (CN) and flash point, has inherent lubricity and demonstrates more diminution in emissions, when compared with fossil diesel.1,2 Jatropha curcas and Alexandrian laurel can be regarded as potential feedstock for biodiesel production because of their non-edible origin, higher oil yield than other non-edible feedstocks and the compliance of the biodiesel yield from its crude oil with the US ASTM D6751 and European Union EN 14214 biodiesel standards.3,4 GTL fuel can be synthesized in a number of ways, such as, the methane reforming process, Fischer–Tropsch (FT) synthesis, and hydrocracking processes.5,6 The FT synthesis converts a mixture of carbon monoxide and hydrogen into various liquid hydrocarbons, by using suitable catalysts.7 GTL fuel possesses higher CN, virtually no sulfur and negligible amounts of aromatics8–10 and also demonstrates significantly lower emission than diesel and biodiesel.11–13 Thus, it can be regarded as a clean alternative fuel that can yield low exhaust emissions, without any major engine modifications and significant loss in efficiency. Previous studies regarding the engine performance-emission tests of alternative fuels like biodiesel, GTL and their blends with diesel, were only confined to some common test conditions, such as, full load or medium load and variation of engine speeds. Most of the studies14–19 showed that biodiesel blends with diesel showed decreasing power, CO, HC and smoke emissions, whereas an increase was observed in fuel consumption and NOx emissions. The research8–10,20 in GTL–diesel blends reported that blending GTL fuel with diesel can certainly improve the fuel properties of the blends, which lead towards better engine performance and exhaust emission than diesel.The effect of idling conditions on alternative fuels at major engine performance-emission parameters should also be considered as a significant research scope, which has not yet been investigated thoroughly. Idling refers to the incessant operation of the primary propulsion engine of any automobile during its stationary position. Generally, automotive engines switch to idling modes in traffic ambiences, particularly at traffic signals and intermittent driving during traffic jam in urban areas. However, the duration of engine idling in these conditions is short compared to the idling state of heavy-duty diesel engines whilst parked during journey intervals on highways with running engines to utilize the air conditioning. Idling results in emission of harmful environmental pollutants and also a significant increase in fuel consumption. A number of studies21–24 on the idling effects on diesel fuel have been reported in the last decades. As the blends of alternative fuel and diesel are being considered as prospective transportation fuels, a comparative analysis of these blends will be of great significance to study their performances at high idling conditions. When the engine is operated at low load-low rated speed conditions, it is regarded as high idling condition.25 At these conditions, the engine cannot attain the required operating temperature, which leads to incomplete combustion. Thus, higher fuel consumption, associated with an increase in exhaust emissions have been observed in idling conditions. Regarding the high idling test conditions, only three studies26–28 have been reported using diesel–biodiesel blends, but no study with GTL–diesel blends have been performed till now. The previous studies had some limitations. The comparative analysis of multiple alternative fuel blends were not illustrated. Besides, those studies did not include smoke emissions at high idling conditions.
1.2. Objective
The objective of this study is to investigate the idling effects of the six blends of JBD, ALBD and GTL fuel with diesel in the context of different engine performance-emission parameters at four different engine test conditions. This study of the idling effects on alternative fuel blends will provide a justification in commercial application of these fuels.2. Experimental set up and procedures
2.1. Biodiesel production
The production process of biodiesel from crude oil depends on the free fatty acid (FFA) content of the crude oil. High FFA content leads to the occurrence of soap formation, which not only constrains the isolation process of the ester from glycerin, but also decreases the transformation rate of ester. According to Canakci and Van Garpen,29 oils with FFA higher than 1%, which illustrates the acid value of 2 mg KOH per g is not susceptible to the alkaline-catalyzed trans-esterification process. Jatropha curcas and Alexandrian laurel crude oil have an FFA content of 8% (acid value 16 mg KOH per g) and 20% (acid value 40 mg KOH per g), respectively. So, acid-catalyzed esterification should be applied at first for both of these crude oils to reduce the FFA level.30 After that the alkali-catalyzed trans-esterification process can be applied. At first, crude oil was placed in a jacket reactor of 1 liter capacity, equipped with an IKA Eurostar digital model stirrer and Wiscircu water bath arrangement. Next, methanol (in 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) and H2SO4 (1% v/v) were added. The system was kept at a temperature of 50–60 °C for 3 hours by circulation of hot water through the jacket, while stirring at a constant speed of 1100 rpm. To check the change in FFA level, a 5 mL sample was collected at regular 10 minute intervals and the process was continued until the FFA level had decreased by 1–2%. After the completion of acid esterification, the mixture was poured into a separation funnel to isolate the esterified product and catalyst layer. The catalyst layer contained excess H2SO4 and alcohol, and was found to be the upper layer. After isolation, the lower layer was extracted and placed into a rotary evaporator to eliminate excess methanol and water. The yield from this process is approximately 98%.31 After the completion of acid esterification, the product was subjected to alkali-catalyzed trans-esterification.
1 molar ratio) and H2SO4 (1% v/v) were added. The system was kept at a temperature of 50–60 °C for 3 hours by circulation of hot water through the jacket, while stirring at a constant speed of 1100 rpm. To check the change in FFA level, a 5 mL sample was collected at regular 10 minute intervals and the process was continued until the FFA level had decreased by 1–2%. After the completion of acid esterification, the mixture was poured into a separation funnel to isolate the esterified product and catalyst layer. The catalyst layer contained excess H2SO4 and alcohol, and was found to be the upper layer. After isolation, the lower layer was extracted and placed into a rotary evaporator to eliminate excess methanol and water. The yield from this process is approximately 98%.31 After the completion of acid esterification, the product was subjected to alkali-catalyzed trans-esterification.
In the alkali-catalyzed trans-esterification process, methanol (25% v/v oil) and potassium hydroxide 1% (w/w oil) were added, with the esterified oil, to the biodiesel reactor. This mixture was stirred at 60 °C for 3 hours, maintaining a speed of 1000 rpm. After that the mixture was transferred to a separation funnel, where it appeared as two layers of glycerol impurities (lower layer) and methyl esters (upper layer). Eventually, the lower layer was isolated and the upper layer was cleansed thoroughly by spraying 50% (v/v) hot (50 °C) distilled water over the methyl esters while shaking gently to ensure proper washing. The cleansing process was repeated until the methyl ester achieved a pH value of 7. Excess water and methanol content were removed from the methyl ester by using an IKA RV10 rotary evaporator. For moisture removal from the methyl ester, anhydrous sodium sulfate was used. Finally, the methyl ester was obtained by filtration using high quality filter papers.
2.2. GTL production process
GTL production stages can be sub-categorized as formation of synthesis gas (syngas), catalytic synthesis (conversion of syngas) and the post processing (cracking). Syngas can be formed from any carbonaceous elements such as: natural gas, petroleum coke coal or biomass. At first, carbon and hydrogen are differentiated from methane. After that, they are transformed in several processes available for syngas production depending on the feed stock, such as partial oxidation, steam reforming, auto thermal reforming (ATR), gasification and a combination of those, which result in different ratios of hydrocarbon–carbon monoxide. In a catalytic synthesis process, the gaseous mixture of CO and H2 (syngas) is processed in various Fischer–Tropsch reactors and yields long-chain, waxy hydrocarbons and a considerable quantity of water as by-product. The reactors used in catalytic synthesis are specified by different designs targeting the technology to produce a wide ranges of paraffinic long-chain hydrocarbons (synthetic crude). Finally, this synthetic crude is processed through traditional refinery cracking operations in the presence of zeolite catalysts and hydrogen to yield catalytically cracked shorter hydrocarbons. These shorter hydrocarbons undergo distillation to produce GTL fuels.322.3. GC analysis and fatty acid composition of fuels
Gas chromatographic (GC) analysis was performed to investigate the fatty acid composition of the produced biodiesel. A sample of 1 μL was injected into a Shidmazu GC-2010A series chromatography panel. The specifications of the instrument and operation conditions are presented in Table 1. The fatty acid composition of the produced biodiesel fuels is presented in Table 2. The test results showed that JBD contains saturated methyl esters (ca. 24.3%), mono-saturated methyl esters (ca. 42.6%), and poly-unsaturated methyl esters (ca. 33.1%).| Property | Specifications |
|---|---|
| Carrier gas | Helium, 83 kpa (split ratio 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| GC column | BPX70, capillary type (30.0 m × 0.25 μm × 0.32 mm, inner diameter) |
| GC column flow rate | 1.10 mL min−1 |
| Injector | Split/split less (type 1177) with EFC control |
| Injection volume | 1 μL |
| Detector type | Flame ionization detector (FID), 250.0 °C |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Operating temperatures | |
| Oven | 140.0 °C |
| Injector | 240.0 °C |
| Detector ports | 260.0 °C |
| Initial hold | 140.0 °C (hold for 2 minutes) |
| Temperature ramp (after initial hold) | 8 °C min−1 to 165.0 °C |
| 3 °C min−1 to 192.0 °C | |
| 8 °C min−1 to 220.0 °C | |
| Fatty acid ester | Structure | Molecular mass | Formula | ALBD | JBD |
|---|---|---|---|---|---|
| Methyl myristate | 14:00 | 242.40 | CH3(CH2)12COOCH3 | 0 | 0.1 |
| Methyl laureate | 12:00 | 214.34 | CH3(CH2)10COOCH3 | 0 | 0.1 |
| Methyl palmitate | 16:00 | 270.45 | CH3(CH2)14COOCH3 | 14.8 | 17.7 |
| Methyl palmitoleate | 16:01 | 268.43 | CH3(CH2)5CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)7COOCH3 CH(CH2)7COOCH3 |
0.3 | 0.8 |
| Methyl stearate | 18:00 | 298.50 | CH3(CH2)16COOCH3 | 16 | 6.4 |
| Methyl oleate | 18:01 | 296.49 | CH3(CH2)7CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)7COOCH3 CH(CH2)7COOCH3 |
41.3 | 41.8 |
| Methyl linoleate | 18:02 | 294.47 | CH3(CH2)3(CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)2(CH2)7COOCH3 CH)2(CH2)7COOCH3 |
26.6 | 32.9 |
| Methyl linolenate | 18:03 | 292.46 | CH3(CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)3(CH2)7COOCH3 CH)3(CH2)7COOCH3 |
0.2 | 0.2 |
| Methyl arachidate | 20:0 | 326.56 | CH3(CH2)18COOCH3 | 0.8 | 0.1 |
| Methyl erucate | 22:1 | 338 | CH3(CH2)7CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)11COOH CH(CH2)11COOH |
0.5 | 0 |
| Methyl eicosenoate | 20:01 | 324.54 | CH3(CH2)16CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH3 CHCOOCH3 |
0.2 | 0 |
| Methyl behenate | 22:00 | 354.62 | CH3(CH2)20COOCH3 | 0 | 0 |
| Methyl lignocerate | 24:00 | 382.66 | CH3(CH2)22COOCH3 | 0 | 0 |
2.4. Fuel blend preparation and properties analysis
In this study, six blends were prepared as sample fuels. JBD, ALBD and GTL fuels were mixed with diesel in two ratios, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and 1
9 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The sample blend with 10% (by vol) of JBD, ALBD or GTL fuel with 90% diesel were named J10, AL10 and G10, respectively. Similarly, the blends containing 20% biodiesel or GTL fuel and 80% diesel were named J20, AL20 and G20, respectively. While preparing the blend, the calculated volumes of diesel and other test fuel were first taken into a sealed magnetic stirrer and next in a shaker. Each of the test sample fuel blends was stirred at 4000 rpm for 30 minutes. Then the stirred blend was placed in the digital shaker for additional 30 minutes at 400 rpm. Finally, the blend sample was removed from the shaker and observed for 12 h to ensure that no phase separation was occurring. The apparatus for fuel property analysis are shown in Table 3. The fuel properties of the produced biodiesel are listed in Table 4. In this table, saponification number (SN), iodine value (IV) and cetane number (CN) were calculated by using the fatty acid composition results and the following empirical eqn (1)–(3),33 respectively.
4. The sample blend with 10% (by vol) of JBD, ALBD or GTL fuel with 90% diesel were named J10, AL10 and G10, respectively. Similarly, the blends containing 20% biodiesel or GTL fuel and 80% diesel were named J20, AL20 and G20, respectively. While preparing the blend, the calculated volumes of diesel and other test fuel were first taken into a sealed magnetic stirrer and next in a shaker. Each of the test sample fuel blends was stirred at 4000 rpm for 30 minutes. Then the stirred blend was placed in the digital shaker for additional 30 minutes at 400 rpm. Finally, the blend sample was removed from the shaker and observed for 12 h to ensure that no phase separation was occurring. The apparatus for fuel property analysis are shown in Table 3. The fuel properties of the produced biodiesel are listed in Table 4. In this table, saponification number (SN), iodine value (IV) and cetane number (CN) were calculated by using the fatty acid composition results and the following empirical eqn (1)–(3),33 respectively.
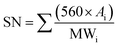 | (1) |
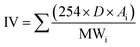 | (2) |
 | (3) |
| Properties | Equipment | Manufacturer | Standard method | ASTM D6751 limit | Accuracy |
|---|---|---|---|---|---|
| Kinematic viscosity | SVM 3000-automatic | Anton Paar, UK | D445 | 1.9–6.0 | ±0.35% |
| Density | SVM 3000-automatic | Anton Paar, UK | Not specified | ±0.1 kg m−3 | |
| Flash point | Pensky-Martens flash point-automatic NPM 440 | Normalab, France | D93 | 130 min | ±0.1 °C |
| Calorific value | C2000 basic calorimeter-automatic | IKA, UK | D240 | D6371 | ±0.1% of reading |
| Oxidation stability | 873 Rancimat—automatic | Metrohm, Switzerland | D675 | 3 hour | ±0.01 hour |
| Cloud point | Cloud and pour point tester—automatic NTE 450 | Normalab, France | D2500 | Report | ±0.1 °C |
| Pour point | Cloud and pour point tester—automatic NTE 450 | Normalab, France | D2500 | Not specified | ±0.1 °C |
| CFPP | Cold filter plugging point—automatic NTL 450 | Normalab, France | D6371 | Not specified | ±0.1 °C |
| Acid value | G-20 Rondolino automated titration system | Mettler Toledo, Switzerland | D664 | 0.5 max | ±0.001 mg KOH per g |
| Properties | ASTM D6751 | EN 14214 | Crude jatropha oil | JBD | Crude AL oil | ALBD |
|---|---|---|---|---|---|---|
| Density@40 °C (g cm−3) | Not specified | 860–900 | 918.9 | 878.8 | 921.6 | 877.0 |
| Kinematic viscosity@40 °C (mm2 s−1) | 1.9–6.0 | 3.5–5.0 | 34.072 | 4.2684 | 53.136 | 5.6872 |
| Flash point (°C) | >130 | >120 | 210.5 | 176.5 | 218.5 | 141.5 |
| Calorific value (MJ kg−1) | Not specified | Not specified | 39.420 | 40.899 | 38.51 | 39.39 |
| Cetane no. | ≥47 | >51 | — | 53.5 | — | 56.3 |
| CP, (°C) | Report | Not specified | 12 | 3 | 8 | 7 |
| PP, (°C) | Not specified | Not specified | 1 | 2 | 8 | 7 |
| CFPP, (°C) | Not specified | Not specified | 22 | 1 | 27 | 8 |
| Acid value (mg KOH per g) | <0.50 | <0.50 | 16 | 0.18 | 40 | 0.34 |
| Saponification number | Not specified | Not specified | — | 192.6 | — | 191.6 |
| Iodine value | Not specified | Not specified | — | 93.8 | — | 82.1 |
| Oxidation stability at 110 °C, (h) | >3 | >6 | 1.2 | 8.41 | 2.43 | 3.58 |
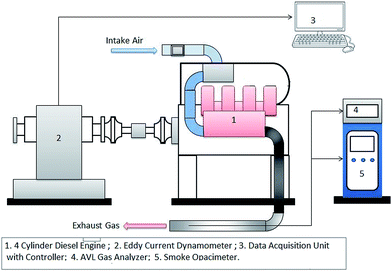
2.5. Engine test rig
A four-cylinder, four-stroke, water-cooled diesel engine was used for experimental investigation. The test engine was directly coupled to a Froude-Hoffman AG250 eddy current dynamometer. The test rig schematic is shown in Fig. 1. The specifications of the test engine and experimental conditions are shown in Table 5. The initial engine run was performed with diesel before starting the tests with all sample fuels. Before starting the test with sample test fuels, the engine was run for ten minutes to ensure removal of any residual diesel. After the test of each sample fuel, the fuel line was purged with diesel to remove that sample and to make it ready for the next sample. This procedure had been maintained for testing in all test conditions. The operations were performed at the same injection timing for all tested fuels.| Engine type | 4 Stroke diesel engine |
|---|---|
| Number of cylinders | 4 in-line, longitudinal |
| Cylinder bore × stroke | 91.1 × 95 mm |
| Displacement | 2477 cm3 |
| Compression ratio | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Combustion chamber | Swirl type |
| Rated power | 65 kW at 4200 rpm |
| Torque | 185 Nm, at 2000 rpm |
| Valve mechanism | Single overhead camshaft (SOHC) |
| Injection pressure (kg cm−2) | 157 bar |
| Aspiration | Turbo charged |
| Fuel system | Distributor type injection pump |
| Cooling system | Radiator cooling |
| Lubrication system | Pressure feed, full flow filtration |
| Test conditions | Specifications |
|---|---|
| 10% load-1000 rpm | TC1 |
| 15% load-1000 rpm | TC2 |
| 10% load-1500 rpm | TC3 |
| 15% load-1500 rpm | TC4 |
In this study, four sets of test conditions were selected. At first, the engine test was performed at 10% load at 1000 rpm and 1500 rpm speed. Then at 15% load at 1000 rpm and 1500 rpm speed. To maintain accuracy, each test point was repeated thrice and the mean value was obtained to plot graphs. Each set of test conditions was given a name for convenience and these are shown in Table 5. In addition, each and every test data series (i.e. test point with the same fuel type and at various engine conditions) was recorded on the same day to minimize substantial day-to-day variation in the experimental results. To measure the fuel flow rate, a positive-displacement type flow meter (KOBOLD ZOD) was installed. For recording the engine test data a REO-dCA data acquisition system was incorporated. For exhaust emission analysis, an AVL DICOM 4000 gas analyzer was used to measure the concentration of CO, HC and NOx. Smoke opacity measurements were recorded with an AVL Di-Smoke 4000. The measurement range and resolution for both of the instruments are given in Table 6.
| Method | Measured component | Range | Resolution |
|---|---|---|---|
| AVL exhaust gas analyser | |||
| Non-dispersive infrared | CO | 0.10% vol | 0.01 vol% |
| Non-dispersive infrared | Unburned HC | 0–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm vol 000 ppm vol |
1 ppm |
| Electrochemical | NOx | 0–5000 ppm vol | 1 ppm |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Smoke opacimeter | |||
| Photodiode detector | Opacity% | 0–100% | 0.10% |
2.6. Accuracies and uncertainty analysis
Table 7 provides the accuracy values of the measured quantities that were used during the experiment. Uncertainties in any experimental procedure occur depending on the experimental conditions, instrument calibrations, observation, data input, test assembly, etc. Therefore, uncertainty analysis is a significant technique to validate the accuracy of the experimental results. In this experiment, percentage uncertainty of all measured quantities were computed by considering the percentage uncertainties of the equipment. Besides, the relative uncertainty of BSFC was determined by means of the linearized approximation method of uncertainty.34 Uncertainty percentages are also shown in Table 7.| Measured components | Measurement techniques | Measuring range | Accuracy | Uncertainty |
|---|---|---|---|---|
| Load | Strain gauge type load cell | 0–600 N m | ±0.1 N m | |
| Speed | Magnetic pick up type | 0–6000 rpm | ±1 rpm | |
| Time | — | — | ±0.1 s | |
| Fuel flow measurement | Positive displacement gear wheel flow meter | 0.5–36 L h−1 | ±0.04 L h−1 | |
| CO | Non-dispersive infrared | 0–10% by vol | 0.01 vol% | ±0.01 vol% |
| HC | Non-dispersive infrared | 0–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm 000 ppm |
±1 ppm vol | ±1 ppm |
| NOx | Electrochemical | 0–5000 ppm | ±1 ppm vol | ±5 ppm |
| Smoke opacity | Photodiode detector | 0–100% | 0.1% | ±0.5% |
3. Results and discussion
3.1. Fuel property analysis
Fuel property analysis had been conducted as part of the investigation to have a prediction about the quality of sample fuel blends prior to the engine test. Table 8 features fuel properties of the fuel blends. All of the blends used in this experiment also meet the ASTM D7467 specification. Excessive density of any fuel results in higher viscosity, which has significant influence in spray atomization efficiency, resulting in poor combustion with formation of engine deposits and higher exhaust emissions.9 Among the sample fuels, all biodiesel blends showed higher density and viscosity, whereas, GTL blends showed lower values of these two parameters, than those of diesel. J10, J20, AL10 and AL20 demonstrated about 4.56%, 9.12%, 8.94% and 12.19% increased kinetic viscosity than diesel, whereas, G10 and G20 showed about 1.15% and 1.64% lower values than diesel. Lower kinematic viscosity of fuel ensures less resistance while flowing through the fuel system and it also leads to better fuel atomization.35| Properties | Diesel | J10 | J20 | AL10 | AL20 | G10 | G20 |
|---|---|---|---|---|---|---|---|
| Density kg m−3 | 829.6 | 832.6 | 835.1 | 835.3 | 840.1 | 821.83 | 815.8 |
| Kinematic viscosity at 40 °C (mm2 s−1) | 3.075 | 3.21 | 3.35 | 3.45 | 3.85 | 3.045 | 3.025 |
| Calorific value | 44.46 | 43.96 | 43.406 | 43.88 | 43.354 | 44.816 | 45.026 |
| Cetane number | 49 | 56 | 58 | 53 | 56 | 59 | 65 |
| Flash point (°C) | 69.5 | 75.5 | 79.5 | 71.5 | 76.5 | 73.5 | 83.5 |
| Oxidation stability at 110 °C (h) | 59.1 | 39.12 | 36.75 | 17.1 | 13.55 | 44.12 | 48.25 |
The flash point maintains an inverse relation to fuel volatility.36 A higher flash point ensures safety of fuel for handling, storage and prevention from unexpected ignition during combustion. J10, J20, AL10, AL20, G10 and G20 demonstrated higher flash points of about 8.63%, 14.38%, 2.88%, 10.1%, 5.76% and 20.14%, respectively, than diesel. In the case of the calorific value, J10, J20, AL10 and AL20 demonstrated about 1.13%, 2.37%, 1.3% and 2.49% lower values than diesel, whereas, G10 and G20 showed higher values by about 0.82% and 1.28% compared to diesel. The higher calorific value of any fuel is desired because it favors the heat release during combustion and improves engine performance.9
CN is a measure of a fuel’s auto-ignition quality characteristics. J10, J20, AL10, AL20, G10 and G20 demonstrated higher CN by about 14.3%, 18.36%, 8.16%, 14.3%, 20.4% and 32.65%, respectively, compared to diesel.
The oxidation stability values for J10, J20, AL10, AL20, G10 and G20 were 39.12 h, 36.75 h, 17.1 h, 13.55 h, 44.12 h and 48.25 h, respectively, which meet the ASTM D7467 specification.
3.2. Engine performance test
The improvement in BSFC for GTL blends can be illustrated by the combustion phenomena and fuel characteristics. For fuel delivered on a fixed volumetric basis, the amount of fuel injected in a single stroke was same for all fuels. Since the GTL blends contain a higher calorific value, it required comparatively a small quantity of fuel per stroke to produce the same power compared to biodiesel blends.37,38 Besides, GTL blends could have demonstrated lower in-cylinder pressure and lower pressure rise rate, which assisted in compensating mechanical losses, resulting better combustion.39 The higher BSFC values of the four biodiesel blends can be ascribed to the combined effect of lower calorific values and higher kinematic viscosity, which resulted in higher fuel consumption to produce the same output power in the constant fuel injection system, when compared to GTL blends and reference fuel diesel. Several studies16,40 also confirmed that the fuel consumption of blends of diesel is increased with the decrease of the calorific value.
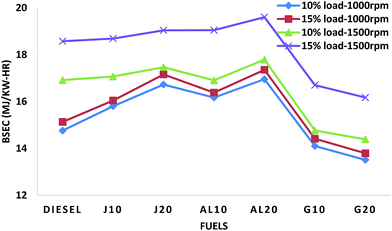
The brake specific energy consumption (BSEC) is introduced to compare the performance of the fuels with different calorific values. It can be defined as the product of the BSFC and calorific value of the fuel. It indicates the amount of energy consumed to produce a unit output power in one hour. In this investigation, the sample fuels have different calorific values, so BSEC is a significant parameter to study the engine performance characteristics. Fig. 3 illustrates the variation of the BSEC values of all fuel samples. It was observed that in all test conditions biodiesel blends showed higher BSEC values, while the GTL blends showed lower values, compared to diesel. On average, J10, J20, AL10 and AL20 showed higher BSEC values by about 3.41%, 7.66%, 4.77% and 9.67%, whereas, G10 and G20 showed lower values by approximately 8.27% and 11.51%, respectively, when compared to those of diesel.
It was observed in all test conditions that both BSFC and BSEC values increase with the increase of quantity of biodiesel in blends, whereas, and GTL blends showed improvement in both BSFC and BSEC values with addition of GTL in blends. It was also observed that at higher speed test conditions (TC3 and TC4), the rate of increase of the BSFC and BSEC values for the biodiesel blends was lower than the lower speed test conditions (TC1 and TC2). This can be attributed to the higher oxygen content of biodiesel which facilitates better combustion at high temperature at high speed condition. The improvement of BSEC of J10–J20 blends compared to AL10–AL20 blends can be ascribed to the higher calorific values of JBD blends than that of ALBD blends. As described earlier, the G10–G20 blends were the best in terms of the improvement of BSFC and BSEC.
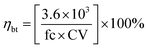 | (4) |
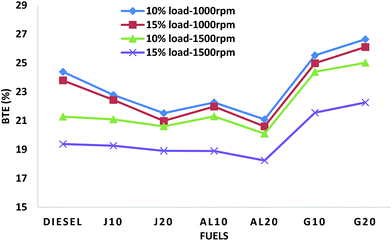
Fig. 4 illustrates the variation of the BTE values of all fuels. It was observed in all test modes that the biodiesel blends showed lower BTE values, while, GTL blends showed higher BTE values than diesel. It was also observed that in TC1 and TC2 the rate of decreasing BTE of the biodiesel blends with addition of biodiesel in blends is much higher than TC3 and TC4. This can be explained by the decrease of calorific values of fuel blends with addition of biodiesel quantity in blends. As described in Section 3.2.1, a higher fuel consumption is required to achieve the same output power and overcome the engine-oriented mechanical loses for fuel blends with lower caloric values. Thus, the BTE decreased significantly from J10 to J20 and AL10 to AL20 in TC1 and TC2. At higher speed conditions (TC3, TC4), a higher level of spontaneous premixing occurs at the top dead center, which induces a faster rate of combustion and thus the effect of decreasing calorific value was not as significant as at lower speed test conditions.39 On average, J10, J20, AL10 and AL20 showed lower BTE by about 3.68%, 7.68%, 4.96% and 9.93%, whereas, G10 and G20 showed higher BTE by approximately 8.56% and 12.58%, respectively, when compared to those of diesel.
3.3. Exhaust emission test
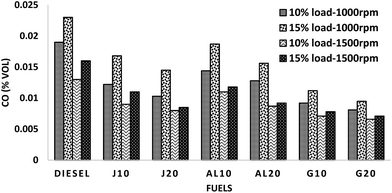
The mysteries of CO emission reduction of GTL blends can be explained by looking at the fuel properties and combustion phenomena. Significant fuel characteristics of GTL, like higher hydrogen–carbon ratio, higher CN and very low aromatic content assist in improved combustion and thus cause reduction of CO emission.9 The higher CN of G10–G20 could have induced shortening of ignition delay that resulted less number of over-lean zones. Besides, the lower distillation temperature of GTL fuel induces rapid vaporization, which reduces the probability of flame quenching and thus ensures lower CO emission.39,42 In case of the other four blends, lower CO emissions can be explained by the combined effect of the higher oxygen content and higher CN.43 Higher CN results short ignition delay, leading towards better combustion. Moreover, the short ignition delay can also be induced by a longer chain length of biodiesel and thus improves combustion process.1 High oxygen content ensures proper in-cylinder temperature, which also facilitates complete combustion.44,45
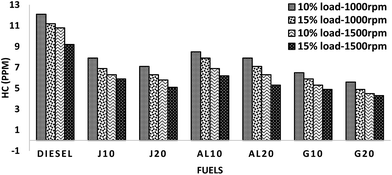
Like for CO emission, reduction of HC emission can be explained using the same parameters. The higher CN of G10–G20 fuel blends shortens the ignition delay, which prevents the formation of the over-lean regions. Lower distillation temperature of GTL ensures a proper pace of evaporation and mixing with air to constitute a more effective combustible charge, which results in less unburned HC in the exhaust emission.42,46 In case of the other four blends, the inherent higher oxygen content of biodiesel induced some advantageous conditions, such as, post-flame oxidation, higher flame speed, etc. throughout the air–fuel interactions, especially in the fuel-rich regions, which ensured the proper oxidation of the unburned HC and thus resulting in significant HC emission reduction.47
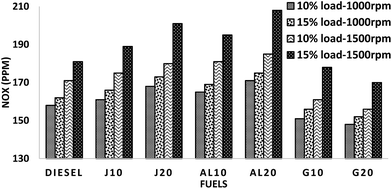
Fig. 7 illustrates the variation of the emission values of all fuels. It was observed in all test modes that J10–J20 and AL10–AL20 blends demonstrate higher NOx emission, whereas G10–G20 showed lower NOx emission values, when compared to diesel. On average, J10, J20, AL10 and AL20 showed higher NOx emission by about 2.83%, 7.44%, 5.65% and 9.81%, whereas, G10 and G20 showed lower NOx emission by approximately 3.89% and 6.85%, respectively, when compared to those of diesel.
The diminution of NOx emission of G10–G20 can be illustrated by the influence of fuel properties in combustion phenomena and exhaust emission. The higher CN of G10–G20 induced shorter ignition delay, followed by a lesser premixed charge, which resulted in a lower combustion temperature and pressure.39,42 It led towards less thermal NOx formation. Significant lower aromatic contents of GTL fuel also influenced G10–G20 blends, which prompted to maintain a lower local adiabatic flame temperature and thus assists in NOx reduction.37,49 Several research studies had revealed an increase of NOx emission in biodiesel or diesel–biodiesel blends with the increase in unsaturation percentage and on the diminution of the chain length.50,51 In the case of the other four blends, higher NOx was observed in all test modes because of their high oxygen content and a higher “premixed part” during combustion, where NOx is primarily formed.52
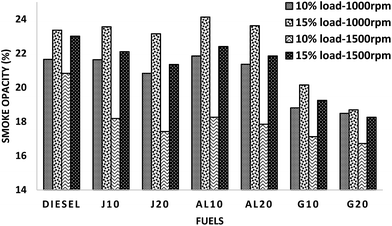
This reduction in smoke emissions in GTL blends can be explained by the combined effect of the absence of aromatics (regarded as soot predecessors), low sulfur content and higher hydrogen to carbon ratio of GTL fuel.2,39 The diminution of the smoke for biodiesel blends can be attributed to the higher oxygen content, associated with lower sulfur content and impurities.53
4. Conclusion
In this study, production of ALBD and JBD was performed. Six blends of J10, J20, AL10, AL20, G10 and G20 were used in a comparative investigation in terms of the fuel properties, engine performance and exhaust emission than diesel. Four engine test conditions were selected for the in-depth analysis of these blends.• The produced ALBD and JBD showed improvement of fuel properties compared to those of their crude oil. The biodiesel blends showed further improvement in properties like density, kinematic viscosity, calorific value, oxidation stability, etc. compared to neat biodiesel. Unlike, diesel–biodiesel blends, GTL–diesel blends showed improvement of fuel properties, such as, lower density and kinematic viscosity, but higher calorific values and cetane number, with increased quantity of GTL in blends.
• It can be deduced from the engine performance-emission results of all sample fuel blends that all of them are eligible for running on unmodified C.I. engines.
• The engine performance test result with all four test conditions showed that G10–G20 showed higher BTE (ca. 8.56–12.58%), whereas, lower BSFC and BSEC (ca. 8.65–12.26% and 8.27–11.51%, respectively), compared with those of diesel. On average, J10–J20 and AL10–AL20 demonstrated higher BSFC (ca. 5.01–10.71% and 6.62–12.18%), higher BSEC (ca. 3.41–7.66% and 4.77–9.67%), whereas, lower BTE (ca. 3.68–7.68% and 4.96–9.93%, respectively), compared to those of diesel.
• Exhaust emission experiments with all four test conditions revealed significant reduction for GTL blends than the other fuels. On average, G10–G20 showed reduction in CO, HC, NOx and smoke emission (ca. 48.25–51.38%, 44.81–51.43%, 3.89–6.85% and 15.21–18.78%, respectively), compared to diesel. On average, J10–J20 and AL10–AL20 demonstrated higher NOx (ca. 2.83–7.44% and 5.65–9.81%), whereas lower CO (ca. 29.12–39.81% and 25.36–33.71%), HC (ca. 33.65–40.27% and 29.67–35.46%), smoke (ca. 3.79–6.87% and 2.49–4.69%, respectively), than those of diesel.
In-detail analysis of the outcome of this study has heightened the possibility of commercial application of all of these alternative fuel blends. These fuel blends may comply with the upcoming strict emission regulation and also contribute to better engine performance features ever cherished by the automobile manufacturers.
Abbreviations
| JBD | Jatropha curcas biodiesel |
| ALBD | Alexandrian laurel biodiesel |
| GTL | Gas-to-liquid fuel |
| BTE | Brake thermal efficiency |
| BSEC | Brake specific energy consumption |
| CO | Carbon monoxides |
| HC | Hydrocarbons |
| NOx | Nitrogen oxides |
| CN | Cetane number |
| FFA | Free fatty acid |
| GC | Gas chromatography |
| J10 | 10% JBD + 90% diesel |
| J20 | 20% JBD + 80% diesel |
| AL10 | 10% ALBD + 90% diesel |
| AL20 | 20% ALBD + 80% diesel |
| G10 | 10% GTL + 90% diesel |
| G20 | 20% GTL + 80% diesel |
| SN | Saponification number |
| IV | Iodine value |
| PPM | Parts per million |
| RPM | Rotation per minute |
| FT | Fischer–Tropsch |
Acknowledgements
The authors would like to acknowledge the University of Malaya for financial support through a High Impact Research grant titled: “Clean Diesel Technology for Military and Civilian Transport Vehicles” having grant number UM.C/HIR/MOHE/ENG/07.References
- J. Xue, T. E. Grift and A. C. Hansen, Renewable Sustainable Energy Rev., 2011, 15, 1098–1116 CrossRef CAS PubMed.
- M. Lapuerta, O. Armas, J. J. Hernández and A. Tsolakis, Fuel, 2010, 89, 3106–3113 CrossRef CAS PubMed.
- M. Mofijur, H. Masjuki, M. Kalam and A. Atabani, Energy, 2013, 55, 879–887 CrossRef CAS PubMed.
- P. Rao, World academy of science, engineering and technology, 2011, vol. 75, pp. 855–868 Search PubMed.
- O. P. R. van Vliet, A. P. C. Faaij and W. C. Turkenburg, Energy Convers. Manage., 2009, 50, 855–876 CrossRef CAS PubMed.
- S. S. Gill, A. Tsolakis, K. D. Dearn and J. Rodríguez-Fernández, Prog. Energy Combust. Sci., 2011, 37, 503–523 CrossRef CAS PubMed.
- O. O. James, B. Chowdhury, M. A. Mesubi and S. Maity, RSC Adv., 2012, 2, 7347–7366 RSC.
- A. J. Velaers and S. d. Goede, SAE Int. J. Fuels Lubr., 2013 DOI:10.4271/2013-01-1136.
- H. Sajjad, H. H. Masjuki, M. Varman, M. A. Kalam, M. I. Arbab, S. Imtenan and S. M. A. Rahman, Renewable Sustainable Energy Rev., 2014, 30, 961–986 CrossRef CAS PubMed.
- S. H. Park, D. Lee and C. S. Lee, Proc. Inst. Mech. Eng., Part D, 2014, 228, 85–93 CrossRef CAS PubMed.
- G. Moon, Y. Lee, K. Choi and D. Jeong, Fuel, 2010, 89, 3840–3846 CrossRef CAS PubMed.
- O. Armas, K. Yehliu and A. L. Boehman, Fuel, 2010, 89, 438–456 CrossRef CAS PubMed.
- M. Oguma, S. Goto, K. Oyama, K. Sugiyama and M. Mori, SAE Tech. Pap. Ser., 2002 DOI:10.4271/2002-01-1706.
- C. Carraretto, A. Macor, A. Mirandola, A. Stoppato and S. Tonon, Energy, 2004, 29, 2195–2211 CrossRef CAS PubMed.
- H. Aydin and H. Bayindir, Renewable Energy, 2010, 35, 588–592 CrossRef CAS PubMed.
- E. Buyukkaya, Fuel, 2010, 89, 3099–3105 CrossRef CAS PubMed.
- J. Song and C. Zhang, Proc. Inst. Mech. Eng., Part D, 2008, 222, 2487–2496 CrossRef.
- M. Gumus and S. Kasifoglu, Biomass Bioenergy, 2010, 34, 134–139 CrossRef CAS PubMed.
- P. Sahoo, L. Das, M. Babu, P. Arora, V. Singh, N. Kumar and T. Varyani, Fuel, 2009, 88, 1698–1707 CrossRef CAS PubMed.
- H. Sajjad, H. Masjuki, M. Varman, M. Kalam, M. Arbab, S. Imtenan and M. Rashed, RSC Adv., 2014, 4, 44529–44536 RSC.
- A. S. Khan, N. N. Clark, M. Gautam, W. S. Wayne, G. J. Thompson and D. W. Lyons, J. Air Waste Manage. Assoc., 2009, 59, 354–359 CAS.
- N. N. Clark, M. Gautam, W. S. Wayne, W. Riddle, R. D. Nine, D. W. Lyons and S. Xu, Examination of a Heavy Heavy-Duty Diesel Truck Chassis Dynamometer Schedule, SAE Tech. Pap. Ser., 2004 DOI:10.4271/2004-01-2904.
- N. Pekula, B. Kuritz, J. Hearne, A. Marchese and R. Hesketh, The effect of ambient temperature, humidity, and engine speed on idling emissions from heavy-duty diesel trucks, SAE Tech. Pap. Ser., 2003 DOI:10.4271/2003-01-0290.
- C.-J. Brodrick, H. A. Dwyer, M. Farshchi, D. B. Harris and F. G. King Jr, J. Air Waste Manage. Assoc., 2002, 52, 1026–1031 Search PubMed.
- S. M. A. Rahman, H. H. Masjuki, M. A. Kalam, M. J. Abedin, A. Sanjid and H. Sajjad, Energy Convers. Manage., 2013, 74, 171–182 CrossRef CAS PubMed.
- M. M. Roy, W. Wang and J. Bujold, Appl. Energy, 2013, 106, 198–208 CrossRef CAS PubMed.
- S. M. A. Rahman, H. H. Masjuki, M. A. Kalam, M. J. Abedin, A. Sanjid and H. Sajjad, Energy Convers. Manage., 2013, 76, 362–367 CrossRef CAS PubMed.
- S. Rahman, H. Masjuki, M. Kalam, M. Abedin, A. Sanjid and M. M. Rahman, Renewable Energy, 2014, 68, 644–650 CrossRef CAS PubMed.
- M. Canakci and J. Van Gerpen, Trans. Am. Soc. Agric. Eng., 2001, 44, 1429–1436 CAS.
- M. Y. Koh and T. I. Mohd Ghazi, Renewable Sustainable Energy Rev., 2011, 15, 2240–2251 CrossRef CAS PubMed.
- I. Rizwanul Fattah, H. Masjuki, M. Kalam, M. Wakil, A. Ashraful and S. Shahir, Energy Convers. Manage., 2014, 83, 232–240 CrossRef CAS PubMed.
- K. Agee, Fundamentals of Gas to Liquids, London: Petroleum Economist, 2nd edn, 2005, pp. 30–31 Search PubMed.
- M. Mohibbe Azam, A. Waris and N. Nahar, Biomass Bioenergy, 2005, 29, 293–302 CrossRef CAS PubMed.
- M. Mofijur, H. H. Masjuki, M. A. Kalam, A. E. Atabani, I. M. R. Fattah and H. M. Mobarak, Ind. Crops Prod., 2014, 53, 78–84 CrossRef CAS PubMed.
- M. I. Arbab, H. H. Masjuki, M. Varman, M. A. Kalam, S. Imtenan and H. Sajjad, Renewable Sustainable Energy Rev., 2013, 22, 133–147 CrossRef CAS PubMed.
- M. Arbab, H. Masjuki, M. Varman, M. A. Kalam, H. Sajjad and S. Imtenan, RSC Adv., 2014, 4, 37122–37129 RSC.
- A. Abu-Jrai, A. Tsolakis, K. Theinnoi, R. Cracknell, A. Megaritis, M. L. Wyszynski and S. E. Golunski, Energy Fuels, 2006, 20, 2377–2384 CrossRef CAS.
- K. Yehliu, A. L. Boehman and O. Armas, Fuel, 2010, 89, 423–437 CrossRef CAS PubMed.
- H. Yongcheng, Z. Longbao, W. Shangxue and L. Shenghua, Proc. Inst. Mech. Eng., Part D, 2006, 220, 827–835 CrossRef.
- M. Lapuerta, O. Armas and J. Rodriguez-Fernandez, Prog. Energy Combust. Sci., 2008, 34, 198–223 CrossRef CAS PubMed.
- J. B. Heywood, Internal Combustion Engine Fundamentals, McGraw-Hill, New York, 2002 Search PubMed.
- T. Wu, Z. Huang, W.-g. Zhang, J.-h. Fang and Q. Yin, Energy Fuels, 2007, 21, 1908–1914 CrossRef.
- J. B. Hirkude and A. S. Padalkar, Appl. Energy, 2012, 90, 68–72 CrossRef CAS PubMed.
- E. Cecrle, C. Depcik, A. Duncan, J. Guo, M. Mangus, E. Peltier, S. Stagg-Williams and Y. Zhong, Energy Fuels, 2012, 26, 2331–2341 CrossRef CAS.
- Y. Di, C. Cheung and Z. Huang, Sci. Total Environ., 2009, 407, 835–846 CrossRef CAS PubMed.
- H. Wang, H. Hao, X. Li, K. Zhang and M. Ouyang, Appl. Energy, 2009, 86, 2257–2261 CrossRef CAS PubMed.
- A. N. Ozsezen, M. Canakci, A. Turkcan and C. Sayin, Fuel, 2009, 88, 629–636 CrossRef CAS PubMed.
- I. M. Rizwanul Fattah, M. A. Kalam, H. H. Masjuki and M. A. Wakil, RSC Adv., 2014, 4, 17787–17796 RSC.
- L. Xinling and H. Zhen, Sci. Total Environ., 2009, 407, 2234–2244 CrossRef PubMed.
- S. K. Hoekman and C. Robbins, Fuel Process. Technol., 2012, 96, 237–249 CrossRef CAS PubMed.
- G. Knothe, Fuel Process. Technol., 2005, 86, 1059–1070 CrossRef CAS PubMed.
- D. Rakopoulos, Fuel, 2013, 105, 603–613 CrossRef CAS PubMed.
- S. Imtenan, M. Varman, H. H. Masjuki, M. A. Kalam, H. Sajjad, M. I. Arbab and I. M. Rizwanul Fattah, Energy Convers. Manage., 2014, 80, 329–356 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |