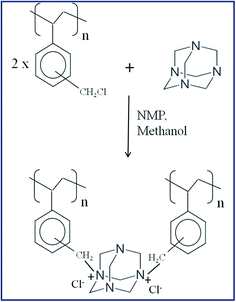
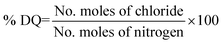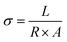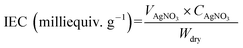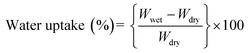Novel cross-linked anion exchange membrane based on hexaminium functionalized poly(vinylbenzyl chloride)
Singaram Vengatesan*,
Subramanyan Santhi,
Ganapathy Sozhan,
Subbiah Ravichandran,
D. Jonas Davidson and
Subramanyan Vasudevan
Electro-Inorganic Chemicals Division, CSIR – Central Electrochemical Research Institute, Karaikudi-630006, Tamilnadu, India. E-mail: svengatesan@cecri.res.in; Fax: +91 4565 227651; Tel: +91 4565 241295
First published on 6th March 2015
Abstract
Hydroxide anion exchange membranes (HAEMs) are of recent research interest, since these membranes can potentially replace the noble metal catalysts used in electrochemical energy conversion systems such as fuel cells and electrolysers. The conductivity and stability of state-of-the-art anion exchange membranes are far below those required for real applications. Herein, we report a novel anion exchange membrane based on aminated and cross-linked poly(vinylbenzyl chloride) prepared by an easy, viable synthetic route. β-Hydrogen free, multi-nitrogen containing ‘hexamethylenetetramine’ was used and explored as an amination/cross-linking agent for the first time in this study. FT-IR and 1H-NMR analysis results confirmed the successful quaternization of poly(vinylbenzyl chloride) with hexamine. TGA results showed the degradation temperature of the quaternized polymer is as high as 160 °C. AFM analysis revealed that the membrane possesses phase separated morphology with hydrophobic and hydrophilic domains. The ionic conductivity of the membranes increased when the amine to polymer ratio was increased from 0.2 to 0.33, and the highest ionic conductivity achieved was 6.8 × 10−3 S cm−1. The membrane has good chemical and alkaline stability which strongly suggests that the membrane would be a promising material for electrochemical energy conversion systems.
1. Introduction
Ion conducting solid polymer membranes find diverse applications in fuel cells, electrolysis, desalination, electrodialysis, etc. Proton exchange membranes (PEMs) are extensively used in fuel cells and electrolysers, as these membranes have high ionic conductivity with good stability. Nevertheless, there are crucial problems, including the usage of expensive noble metal catalysts, perfluorinated membranes, catalyst poisoning, and complex water-management issues.1–4 Hydroxide ion conducting alkaline anion exchange membranes (HAEMs) could unravel many of the limitations of PEM based membranes.5,6 Because electrochemical reactions are more facile in an alkaline medium, non-noble metals can be used as catalysts, thus making the system more cost effective.7–9 HAEMs predominantly contain fixed cationic groups, and mobile negatively charged OH− ions. HAEMs that contain different cationic groups such as ammonium, sulfonium, phosphonium, guanidinium, pyridinium, imidazolium, etc. have been extensively studied.10–19 Among them, quaternary ammonium based HAEMs are used most in electrochemical systems due to their reasonable stability in alkaline conditions. The hydroxide ions move from the cathode to the anode in an operating fuel cell or electrolyser, and the ionic conductivity is a decisive factor to decide the efficiency of these systems. Despite the intense research efforts on anion exchange membranes, their conductivities are not adequately high for realistic applications. One more challenge lies in their low stability under fuel cell working conditions, since the QA group decomposes via Hoffman elimination (due to the presence of β-hydrogens) and/or SN2 substitution reactions in strongly basic environments.20–22 The challenging feature of developing HAEMs is to improve the chemical stability of cationic groups by using QAs with no β-hydrogens. One more strategy for improving the membrane stability is chemical cross-linking of the polymer units.23–27Here, we report the preparation of anion exchange membranes with a cross-linked structure (Scheme 1) by a one-step synthetic route. It mainly involves the preparation of a quaternized poly(vinylbenzyl chloride) (PVBC) anion exchange polymer using an inexpensive amination/cross-linking reagent, hexamethylenetetramine (HMTA). HMTA, commonly known as ‘Urotropine’, is an amine base having a cage-like structure which finds various applications, especially in the plastic and rubber industries as a hardener/binder. In the literature, different amination/cross-linking agents such as ethylene diamine, hexamethylene diamine, tetramethylene pentamine, triethylene diamine have been used and most of them have a β-hydrogen in their structure.28–31 Pandey et al. prepared highly cross-linked anion exchange membranes using 1,4-diazabicyclo[2.2.2]octane as a cross-linker.31 Cao et al. prepared a novel alkaline anion exchange membrane using methylated melamine as an amination/cross-linking agent.32 Though the membrane afforded high ionic conductivity and stability, the methylation step described in their work is a particularly excessive pathway. However, the present work uses the amination agent HMTA, taking advantage of its β-hydrogen free structure which can circumvent the harmful elimination and/or substitution reactions. Moreover, this work involves a single step fabrication of the membrane by casting the viscous solution of the quaternization reaction mixture without further processing or modification.
2. Experimental section
2.1 Materials
Poly(vinylbenzyl chloride), (60/40 mixture of 3- and 4-isomers with an average molecular weight of Mn 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), was purchased from Sigma Aldrich. Hexamethylenetetramine (99% purity) was purchased from Otto Chemika-Biochemika reagents and potassium hydroxide was purchased from Thermo Fischer Scientific India Pvt. Ltd. The other chemicals, namely, methanol, N-methyl pyrrolidone (NMP) and tetrahydrofuran (THF) were of analytical grade and used as received. De-ionised water was used throughout this study.
000), was purchased from Sigma Aldrich. Hexamethylenetetramine (99% purity) was purchased from Otto Chemika-Biochemika reagents and potassium hydroxide was purchased from Thermo Fischer Scientific India Pvt. Ltd. The other chemicals, namely, methanol, N-methyl pyrrolidone (NMP) and tetrahydrofuran (THF) were of analytical grade and used as received. De-ionised water was used throughout this study.
2.2 Amination and membrane preparation
Poly(vinylbenzyl chloride) was dissolved in NMP and, separately, hexamethylenetetramine was dissolved in methanol. The polymer and amine solutions were mixed together and stirred at 60 °C for 30 min. This amination process led to the quaternization of the PVBC polymer. After the amination step, the homogeneous mixture was casted on a glass plate by the solution casting method. Then, the membrane was dried in a vacuum oven at 70 °C for 30 min and further cured at 120 °C for 12 h. The membrane was peeled-off from the glass plate and washed thoroughly with de-ionised water. It was then soaked in 0.5 M KOH for 12 h (to convert the membrane into OH− form). A series of membranes with various HMTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVBC weight ratios (0
PVBC weight ratios (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.2
1, 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.25
1, 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.33
1, 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 0.5
1, and 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were prepared using the above procedure. For comparison, a PVBC membrane without quaternization was also prepared. The thickness of the membranes was 100 ± 5 μm in dry form.
1) were prepared using the above procedure. For comparison, a PVBC membrane without quaternization was also prepared. The thickness of the membranes was 100 ± 5 μm in dry form.
For the solubility test, quaternized anion exchange polymers with HMTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVBC ratios of 0
PVBC ratios of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.2
1, 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.33
1, 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.5
1, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were also synthesized in tetrahydrofuran (replacing NMP and methanol in the previous procedure) at 60 °C.
1 were also synthesized in tetrahydrofuran (replacing NMP and methanol in the previous procedure) at 60 °C.
2.3 Degree of quaternization
The degree of quaternization (DQ) of PVBC with various amounts of HMTA was calculated from the exchangeable chloride content (using argentometric titration), and the total nitrogen content (using CHNS analysis) of the quaternized membrane.332.4 Ionic conductivity
The ionic conductivity (IC) of the membranes in OH− form was measured using AC impedance spectroscopy. To perform the impedance analysis, an electrochemical work station/multi-potentiostat (model: VMP3, Bio-logic SAS) was employed. A home-made four-electrode conductivity cell was used to assemble the membrane test samples.34 The membrane sample was cut into 1 cm × 4 cm and the membrane was clamped between the current (Pd foil) and potential (Pt wire) probes. During the measurement, the conductivity cell was immersed in de-ionised water at 25 °C. The impedance measurements were carried out in galvanostatic mode over the frequency range from 1 MHz to 1 Hz at the amplitude of 1 mA. The in-plane ionic conductivity (σ) was calculated using the eqn:where L (cm) is the distance between the two probes, R (Ω) is the resistance of the membrane, and A is the cross-sectional area [thickness (t) × width (w)].
2.5 Ion-exchange capacity
The ion-exchange capacity (IEC) of the anion exchange membranes was measured using Mohr’s method.35 The dry OH− forms of the membranes were soaked in 0.5 M sodium chloride solution and equilibrated for 24 h at 25 °C. The chloride form of the membrane was converted to a carbon trioxide form by immersion in a 0.5 M sodium carbonate solution. The solution was titrated against 0.1 M silver nitrate using a 5% solution of potassium chromate until a brown sediment precipitated. The ion exchange capacity was calculated using the eqn:where VAgNO3 is the volume of silver nitrate consumed, CAgNO3 is the millimolar concentration of the silver nitrate solution, and Wdry is the mass of the dried membrane (Cl− form) in g.
2.6 Water uptake
The water uptake of the membranes was measured using the following procedure: the anion exchange membrane (OH− form) was cut into a piece (3 × 3 cm) and then soaked in distilled water for 24 h. The membrane’s surface water was removed carefully using a wipe and the wet weight (Wwet) of the membrane was measured. Then, the membrane was dried in a vacuum oven at 60 °C for 24 h. The dry membrane was transferred immediately to a desiccator before cooling to room temperature. The water uptake of the anion exchange membrane was calculated using the eqn:where Wdry is the weight of the dry membrane (g), and Wwet is the weight of the wet membrane (g).
2.7 Stability tests
The chemical stability of the anion exchange membranes was tested in both alkaline solution and Fenton’s solution separately. The alkaline stability of the membrane was carried out by immersing a piece of membrane with a known weight in a 5 M KOH solution for 10 days at 30 °C. The membrane was taken from the KOH solution and rinsed with de-ionised water several times. Then, the ionic conductivity and ion-exchange capacity of the alkaline treated membrane were measured using the standard protocols.The oxidation stability of the membranes was also tested in Fenton’s solution. The Fenton’s solution was prepared using ferrous sulfate (FeSO4) and hydrogen peroxide (H2O2) in the weight ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 at the acidic pH of 3. A membrane sample with a known weight was immersed in the Fenton’s solution for 10 days at 30 °C. Then, the membrane sample was taken from the Fenton’s solution and rinsed with de-ionised water, followed by drying in a vacuum oven at 60 °C for 24 h. From the above experiments, the weight loss of the membrane was calculated.
8 at the acidic pH of 3. A membrane sample with a known weight was immersed in the Fenton’s solution for 10 days at 30 °C. Then, the membrane sample was taken from the Fenton’s solution and rinsed with de-ionised water, followed by drying in a vacuum oven at 60 °C for 24 h. From the above experiments, the weight loss of the membrane was calculated.
2.8 Characterisations
FT-IR spectra of the poly(vinylbenzyl chloride) and quaternized anion exchange polymer were recorded using a Bruker 27 Tensor FT-IR spectrophotometer with a resolution range of 0.125 cm−1. 1H-NMR analysis of the polymerisation products was performed on a Bruker Avance 400 MHz instrument using DMSO-d6 as a solvent and tetramethylsilane (TMS) as an internal reference. The thermal degradation and stability of the membranes were investigated using a simultaneous TGA/DTA analyser (model: SDT Q600, TA Instruments) from 30 to 700 °C at the heating rate of 5 °C min−1 under a nitrogen atmosphere. The nitrogen contents of the quaternized polymers were measured using a CHNS elemental analyser (model: Vario EL III, Elementar, Germany).The surface morphological features of the membrane were analysed using a TESCAN scanning electron microscope (model: S-3000H, Hitachi, Japan). The localization of hydrophilic domains in the membrane was analysed using an atomic force microscope (model: Picoscan 2100, Molecular imaging, USA).
3. Results and discussion
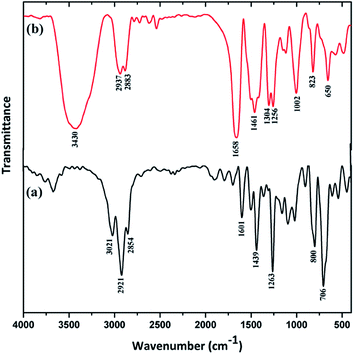
The quaternization of PVBC with HMTA was confirmed using the FTIR spectra shown in Fig. 1.The spectrum of PVBC has an absorption band at 1263 cm−1 that is attributed to CH2Cl wagging,36 and the region of 750–800 cm−1 is assigned to C–Cl stretching.37 The bands at 2921 and 2854 cm−1 are related to symmetric and asymmetric CH2 stretching, and the band at 1601 cm−1 is due to aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching. The quaternized PVBC polymer shows two major peaks at 1002 and 1304 cm−1, which are assigned to C–N stretching vibrations.36 Also, the C–Cl stretching vibration (800 cm−1) is not present in the quaternized PVBC spectrum, confirming the quaternization reaction. The broad peak which appears at 3430 cm−1 is due to O–H vibrations. Additionally, the intense peaks at 1461 and 1658 cm−1 indicate the presence of quaternary ammonium groups as reported in the literature.32 Notably, the intensity of the stretching band at 1658 cm−1 increased in the quaternized PVBC spectrum which might arise from the cross-linking of the PVBC polymer.
C stretching. The quaternized PVBC polymer shows two major peaks at 1002 and 1304 cm−1, which are assigned to C–N stretching vibrations.36 Also, the C–Cl stretching vibration (800 cm−1) is not present in the quaternized PVBC spectrum, confirming the quaternization reaction. The broad peak which appears at 3430 cm−1 is due to O–H vibrations. Additionally, the intense peaks at 1461 and 1658 cm−1 indicate the presence of quaternary ammonium groups as reported in the literature.32 Notably, the intensity of the stretching band at 1658 cm−1 increased in the quaternized PVBC spectrum which might arise from the cross-linking of the PVBC polymer.
Fig. 2 represents the NMR spectra of the PVBC, HMTA and quaternized PVBC. From the NMR spectra, the aliphatic protons (–CH2 and –CH) of PVBC appear at around 1 to 2 ppm, whereas the aromatic ring protons appear at 6.5 and 7.2 ppm. The peak at around 4.6 ppm is assigned to the CH2Cl group. On the other hand, the hexamine spectrum shows a single peak at 4.55 ppm which corresponds to six equivalent methylene (–CH2) protons. The peaks appearing at around 2.5 and 3.3 ppm in all three spectra are the signals from the DMSO-d6 and moisture present in DMSO-d6, respectively. In the case of the quaternized PVBC spectrum, a new peak appears at 5.0 ppm in addition to the peak at 4.6 ppm. The new peak which appears in the downfield region is assigned to the quaternized CH2Cl group. This result confirms the quaternization of the PVBC with hexamine.
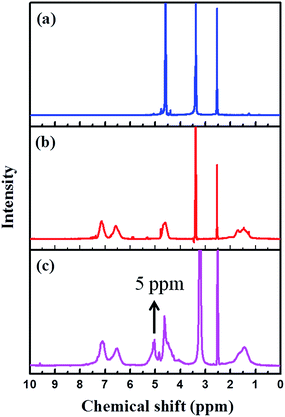 | ||
Fig. 2 1H-NMR spectra of (a) HMTA, (b) PVBC and (c) quaternized PVBC in Cl− form (HMTA/PVBC = 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Fig. 3 shows a photograph of the anion exchange polymers synthesized with different amine/polymer ratios in THF solvent. The solution opacity changes as the amine content is increased in the PVBC polymer, and it converts into a viscous gel (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) and a solid precipitate (1
1 ratio) and a solid precipitate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) at high amine concentrations. This reveals that the PVBC was aminated and cross-linked by HMTA which increased the solution viscosity and reduced the solubility of the quaternized polymer. Besides, cross-linking of the polymer chains would also increase the molecular weight and reduce the solubility of the quaternized polymer. The degree of quaternization of PVBC with various amounts of HMTA was calculated from the chloride content of the membrane. The calculated DQ (%) of the membrane with HMTA/PVBC ratios of 0.2, 0.25, 0.33, and 0.5 are 27.2, 36.3, 47.2 and 61.6%, respectively. From these results, it is clear that the degree of quaternization increases with increasing amounts of HMTA.
1 ratio) at high amine concentrations. This reveals that the PVBC was aminated and cross-linked by HMTA which increased the solution viscosity and reduced the solubility of the quaternized polymer. Besides, cross-linking of the polymer chains would also increase the molecular weight and reduce the solubility of the quaternized polymer. The degree of quaternization of PVBC with various amounts of HMTA was calculated from the chloride content of the membrane. The calculated DQ (%) of the membrane with HMTA/PVBC ratios of 0.2, 0.25, 0.33, and 0.5 are 27.2, 36.3, 47.2 and 61.6%, respectively. From these results, it is clear that the degree of quaternization increases with increasing amounts of HMTA.
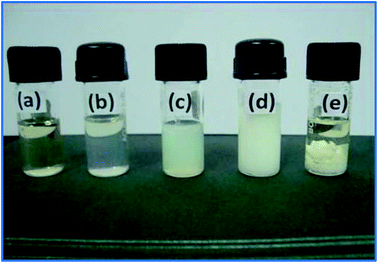 | ||
Fig. 3 Photograph of the quaternized anion exchange polymers with different HMTA/PVBC ratios (a) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (b) 0.2 1; (b) 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (c) 0.33 1; (c) 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (d) 0.5 1; (d) 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (e) 1 1; (e) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in THF. 1 in THF. | ||
Thermo-gravimetric analysis was performed to estimate the thermal stability of the quaternized anion exchange polymer. The TGA curves of the PVBC and quaternized PVBC polymer are shown in Fig. 4. The PVBC polymer shows two major degradation steps: the polymer backbone degradation from 310 °C to 410 °C, and carbonization from 420 °C to 700 °C. However, the quaternized PVBC polymer shows three distinct degradation steps: the quaternary ammonium group degradation from 160 °C to 250 °C, polymer degradation from 310 °C to 400 °C, and carbonization from 410 °C to 700 °C. The TGA results are in good agreement with the literature results reported for anion exchange polymers containing quaternary ammonium groups.32
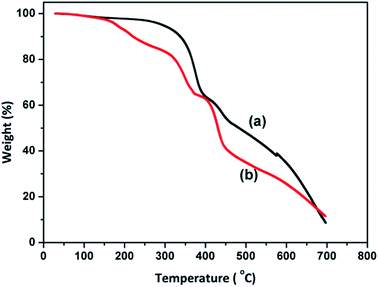 | ||
Fig. 4 Thermo-gravimetric curves of (a) PVBC and (b) quaternized PVBC in Cl− form (HMTA/PVBC = 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
The membranes with different HMTA/PVBC ratios were fabricated using the quaternized PVBC (Cl− form) in NMP solution, except for the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The 1
1. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 membrane could not be achieved due to the formation of a solid precipitate before casting the reaction mixture. The optical image of the anion exchange membrane is depicted in Fig. 5a which displays a yellowish membrane with a smooth surface. Fig. 5b and c are the SEM illustrations of the surface and cross-sectional morphological features of the membrane. The membrane shows no signs of cracks and/or voids. The cross-section of the membrane (Fig. 5c) appears smooth without any perforations or defects.
1 membrane could not be achieved due to the formation of a solid precipitate before casting the reaction mixture. The optical image of the anion exchange membrane is depicted in Fig. 5a which displays a yellowish membrane with a smooth surface. Fig. 5b and c are the SEM illustrations of the surface and cross-sectional morphological features of the membrane. The membrane shows no signs of cracks and/or voids. The cross-section of the membrane (Fig. 5c) appears smooth without any perforations or defects.
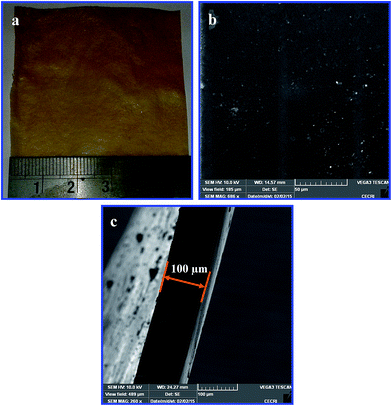 | ||
Fig. 5 (a) Optical image, (b) surface and (c) cross-sectional SEM images of the quaternized PVBC membrane (HMTA/PVBC = 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Fig. 6 shows the ionic conductivity and ion-exchange capacity of the membranes in their OH− form. The PVBC only membrane exhibits very low ionic conductivity in the order of 10−7 S cm−1 (data not shown here). A quantum jump in conductivity is observed once the PVBC is quaternized with HMTA, i.e. 10−7 S cm−1 to 10−3 S cm−1. The conductivity rises exponentially as the HMTA/PVBC ratio is increased from 0.2 to 0.33 which reflects the vital role of the amination agent in the quaternization of the PVBC polymer. However, a further increase in the HMTA/PVBC ratio from 0.33 to 0.5 produces a decrease in conductivity. This observed phenomenon is due to the high level of polymer cross-linking as the amine content was increased to a greater extent. As reported by Xiong et al.,38 a high level of cross-linking reduces the membrane conductivity due to the formation of a membrane compact structure that narrows the anion transfer channels. From the figure, the ion exchange capacity of the membranes also shows a linear increasing trend with the increase in amine/polymer ratios. The IEC increases from 0.99 to 3.42 meq. g−1 (Table 1) as the amine/polymer ratio is increased from 0.2 to 0.5. It is apparent that a greater number of ion exchange groups can be introduced during the quaternization process, since the amination agent HMTA possesses multi-nitrogen centres in its structure. The nitrogen content of the membranes obtained from elemental analysis (Table 1) shows that the membrane acquires a significant amount of nitrogen in its composition, and it increases as the amine to polymer ratio is increased.
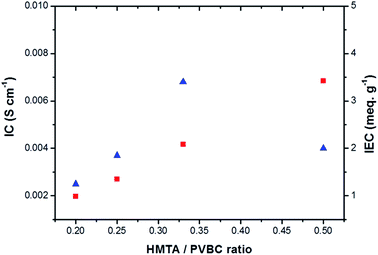 | ||
Fig. 6 Ionic conductivity ( ) and ion-exchange capacity ( ) and ion-exchange capacity ( ) of the anion exchange membranes with different HMTA/PVBC ratios at 25 °C. ) of the anion exchange membranes with different HMTA/PVBC ratios at 25 °C. | ||
In general, ion exchange membranes have the tendency to absorb water, since they possess a high density of hydrophilic ionic groups. Moreover, the water content of the membrane also plays a vital role in the ion conduction process. Therefore, the water uptake of the prepared anion exchange membranes was measured in the OH− form and the results are given in Table 1. The water uptake data of the membranes show that the uptake increases as the amine to polymer ratio is increased. This is mainly attributed to the high level of quaternization as the amine content is increased. The high level of quaternization is likely to introduce a higher number of ionic groups in the anion exchange polymer and increase the water absorption capacity of the membrane.
The chemical stability of a membrane is crucial for long term operations, and the stabilities of the anion exchange membranes were measured under strong alkaline and oxidizing conditions. The membranes were immersed in 5 M KOH and Fenton’s solution separately for 10 days at 30 °C and the stability test results are shown in Table 2. The alkaline stability of the membranes was evaluated in terms of IC and IEC values. From the table, the membrane IC and IEC values decreased very marginally compared to the untreated membranes (Table 1). Notably, the membranes with a high amine concentration (0.33 and 0.5) exhibit low alkaline stability compared to those with a low amine concentration (0.2 and 0.25). Also, after immersing in Fenton’s solution the membranes showed negligible weight loss (∼1%). Fenton’s test was performed especially to see the oxidation stability of the anion exchange membranes in the presence of sensitive OH radicals. These results reveal that the membranes are chemically stable.
To observe the morphological features of the membrane thoroughly, AFM analysis was performed in tapping mode and the results are shown in Fig. 7. The topography image (Fig. 7a) of the membrane shows microphase separated morphology and the microphase separation mainly occurs between the hydrophobic backbone and the hydrophilic graft chains.39 The 3D phase image (Fig. 7b) of the membrane shows the distribution of peak and valley regions. Earlier, Luo et al. studied the poly(vinylbenzyl chloride–methylmethacrylate) copolymer based anion exchange membrane using AFM and identified that the peak regions mostly arise from the hydrophobic domains and the valley regions from the hydrophilic ionic domains.40 It can be predicted from the above results that the membrane developed in the present work possesses the phase separated morphology which is typically found in Nafion® based membranes.41
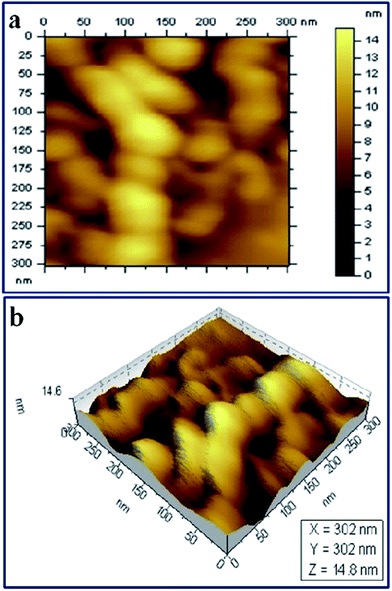 | ||
Fig. 7 AFM tapping mode: (a) topography image and (b) 3D phase image of the anion exchange membrane (HMTA/PVBC = 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
The quaternization of alkyl halides with HMTA was reported earlier by Vidal et al.,42 in the preparation of QA functionalised silica sorbents for solid-phase extraction. However, we report for the first time the simultaneous amination and cross-linking of a chloropolymer using HMTA, and explored the applicability of the QA functionalized polymer as an anion exchange membrane. Moreover, this work is also unique as it involves the preparation an anion exchange membrane in a single step using an inexpensive amination/cross-linking agent. Further, the absence of any β-hydrogen in the amination agent eliminates the possible degradation of the anion exchange membranes by Hoffman elimination and/or substitution reactions. Though the developed membranes were chemically stable, their mechanical flexibility is poor due to the high cross-linking and the rigidity of the aromatic polymer which hampers the application in real systems.
4. Conclusion
A novel anion exchange membrane was successfully prepared using poly(vinylbenzyl chloride) and hexamethylenetetramine as an amination and cross-linking agent. The IC and IEC values of the membranes increased with increase of the amine/polymer ratio which emphasized the role of the amination agent in the architecture of the membrane. The membrane’s water uptake was comparatively low due to the high cross-linking of the polymer units during the quaternization process.TGA results showed the thermoplastic behaviour of the quaternized polymer which mainly occurred due to the incorporation of hydrophilic QA groups into the hydrophobic aromatic polymer backbone. AFM analysis revealed the presence of well defined hydrophobic and hydrophilic phase separation within the membrane. The chemical stability of the membranes was superior in alkaline and oxidizing environments, whereas the mechanical properties have to be improved for practical application of the membranes in real electrochemical systems.
Acknowledgements
The corresponding author S.V. gratefully acknowledges the Director, CSIR-Central Electrochemical Research Institute for providing the financial support through the institute in-house project (project code-OLP 0075).References
- D. J. Berger, Science, 1999, 286, 49–50 CrossRef CAS.
- Q. Li, R. He, J. O. Jensen and N. J. Bjerrum, Chem. Mater., 2003, 15, 4896–4915 CrossRef CAS.
- J. H. Wee and K. Y. Lee, J. Power Sources, 2006, 157, 128–135 CrossRef CAS PubMed.
- H. Li, Y. Tang, Z. Wang, Z. Shi, S. Wu, D. Song, J. Zhang, K. Fatih, J. Zhang, H. Wang, Z. Liu, R. Abouatallah and A. Mazza, J. Power Sources, 2008, 178, 103–117 CrossRef CAS PubMed.
- J. R. Varcoe and R. C. T. Slade, Fuel Cells, 2005, 5, 187–200 CrossRef CAS.
- Y. J. Wang, J. Qiao, R. Baker and J. Zhang, Chem. Soc. Rev., 2012, 42, 5768–5787 RSC.
- Y. Wang, L. Li, L. Hu, L. Zhuang, J. Lu and B. Xu, Electrochem. Commun., 2003, 5, 662–666 CrossRef CAS.
- K. Asazawa, K. Yamada, H. Tanaka, A. Oka, M. Taniguchi and T. Kobayashi, Angew. Chem., Int. Ed., 2007, 46, 8024–8027 CrossRef CAS PubMed.
- S. Shen, T. S. Zhao, J. Xu and Y. Li, Energy Environ. Sci., 2011, 4, 1428–1433 CAS.
- K. Shen, J. Pang, S. Feng, Y. Wang and Z. Jiang, J. Membr. Sci., 2013, 440, 20–28 CrossRef CAS PubMed.
- J. Si, S. Lu, X. Xu, S. Peng, R. Xiu and Y. Xiang, ChemSusChem, 2014, 7, 3389–3395 CrossRef CAS PubMed.
- Z. Zhao, J. Wang, S. Li and S. Zhang, J. Power Sources, 2011, 196, 4445–4450 CrossRef CAS PubMed.
- S. Gu, R. Cai, T. Luo, K. Jensen, C. Contreras and Y. Yan, ChemSusChem, 2010, 3, 555–558 CrossRef CAS PubMed.
- S. Gu, R. Cai and Y. Yan, Chem. Commun., 2011, 47, 2856–2858 RSC.
- B. Zhang, S. Gu, J. Wang, Y. Liu, A. M. Herring and Y. Yan, RSC Adv., 2012, 2, 2683–12685 Search PubMed.
- C. Qu, H. Zhang, F. Zhang and B. Liu, J. Mater. Chem., 2012, 22, 8203–8207 RSC.
- D. S. Kim, A. Labouriau, M. D. Guiver and Y. S. Kim, Chem. Mater., 2011, 23, 3795–3797 CrossRef CAS.
- A. B. Huang, C. Y. Xia, C. B. Xiao and L. Zhuang, J. Appl. Polym. Sci., 2006, 100, 2248–2251 CrossRef CAS.
- B. C. Lin, L. H. Qiu, J. M. Lu and F. Yan, Chem. Mater., 2010, 22, 6718–6725 CrossRef CAS.
- G. Merle, M. Wessling and K. Nijmeijer, J. Membr. Sci., 2011, 377, 1–35 CrossRef CAS PubMed.
- A. C. Cope and A. S. Mehta, J. Am. Chem. Soc., 1963, 85, 1949–1952 CrossRef CAS.
- S. Chempath, B. R. Einsla, L. R. Pratt, C. S. Macomber, J. M. Boncella, J. A. Rau and B. S. Pivovar, J. Phys. Chem. C, 2008, 112, 3179–3182 CAS.
- J. Pan, C. Chen, L. Zhuang and J. Lu, Acc. Chem. Res., 2012, 45, 473–481 CrossRef CAS PubMed.
- J. Pan, Y. Li, L. Zhuang and J. Lu, Chem. Commun., 2010, 46, 8597–8599 RSC.
- J. R. Varcoe, R. C. T. Slade and E. L. H. Yee, Chem. Commun., 2006, 1428–1429 RSC.
- Y. Luo, J. Guo, C. Wang and D. Chu, Electrochem. Commun., 2012, 16, 65–68 CrossRef CAS PubMed.
- J. Fang, Y. Yang, X. Lu, M. Ye, W. Li and Y. Zhang, Int. J. Hydrogen Energy, 2012, 37, 594–602 CrossRef CAS PubMed.
- E. N. Komkova, D. F. Stamatialis, H. Strathmann and M. Wessling, J. Membr. Sci., 2004, 244, 25–34 CrossRef CAS PubMed.
- T. W. Xu and F. F. Zha, J. Membr. Sci., 2002, 199, 203–210 CrossRef CAS.
- W. Lu, Z. G. Shao, G. Zhang, J. Li, Y. Zhao and B. Yi, Solid State Ionics, 2013, 246, 8–18 CrossRef PubMed.
- A. K. Pandey, A. Goswami, D. Sen, S. Mazumder and R. F. Childs, J. Membr. Sci., 2003, 217, 117–130 CrossRef CAS.
- Y. C. Cao, X. Wang, M. Mamlouk and K. Scott, J. Mater. Chem., 2011, 21, 12910–12916 RSC.
- J. Wu, W. Wei, L. Y. Wang, Z. G. Su and G. H. Ma, Biomaterials, 2007, 28, 2220 CrossRef CAS PubMed.
- J. J. Sumner, S. E. Greager, J. J. Ma and D. D. DesMarteau, J. Electrochem. Soc., 1998, 145, 107–110 CrossRef CAS PubMed.
- S. G. Park, N. S. Kwak, C. W. Hwang, H. M. Park and T. S. Hwang, J. Membr. Sci., 2012, 423, 429–437 CrossRef PubMed.
- D. L. Pavia, G. M. Lampman and G. S. Kriz, Introduction to spectroscopy, Thomson Learning, U.S.A, 3rd edn, 2001 Search PubMed.
- Y. J. Choi, M. S. Kang, J. Cho and S. H. Moon, J. Membr. Sci., 2003, 221, 219–231 CrossRef CAS.
- Y. Xiong, J. Fang, Q. H. Zeng and Q. L. Liu, J. Membr. Sci., 2008, 311, 319–325 CrossRef CAS PubMed.
- J. Ran, L. Wu, B. Wei, Y. Chen and T. Xu, Sci. Rep., 2014, 4, 6486 CrossRef PubMed.
- Y. Luo, J. Guo, Y. Liu, Q. Shao, C. Wang and D. Chu, J. Membr. Sci., 2012, 423, 209–214 CrossRef PubMed.
- R. S. McLean, M. Doyle and B. B. Sauer, Macromolecules, 2000, 33, 6541–6550 CrossRef CAS.
- L. Vidal, O. Robin, J. Parshintsev, J. P. Mikkola and M. L. Riekkola, J. Chromatogr. A, 2013, 1285, 7–14 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |