Silver ion post-column derivatization electrospray ionization mass spectrometry for determination of tetrabromobisphenol A derivatives in water samples
Abstract
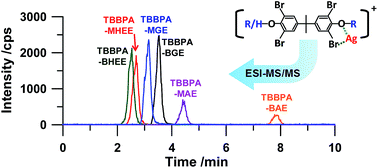
A rapid and sensitive method was developed for the identification and quantitation of tetrabromobisphenol A (TBBPA) derivatives in water samples. Six major TBBPA derivatives, including tetrabromobisphenol A bis(2-hydroxyethyl) ether (TBBPA-BHEE), tetrabromobisphenol A bis(glycidyl) ether (TBBPA-BGE), tetrabromobisphenol A bis(allyl) ether (TBBPA-BAE), tetrabromobisphenol A mono(2-hydroxyethyl) ether (TBBPA-MHEE), tetrabromobisphenol A mono(glycidyl) ether (TBBPA-MGE) and tetrabromobisphenol A mono(allyl) ether (TBBPA-MAE), were selected as the target compounds. By applying the silver cation (Ag+) as the post-column derivatization reagent, the TBBPA derivatives formed complexes ([M + Ag]NO3) online, which could be effectively electrosprayed to generate ionic clusters ([M + Ag]+) for sensitive mass analysis. Under the optimized conditions, the 6 TBBPA derivatives were separated and detected within 10 min. The limits of detection (LODs) were between 0.16 and 1.96 μg L−1, and the linear ranges extended to 200 μg L−1 (R2 ≥ 0.9957). The relative standard deviations (RSDs) were less than 7.7% for 10 μg L−1 of the TBBPA derivatives (n = 7). The proposed method was successfully applied in analysis of environmental water samples. The spiked recoveries ranged from 81.3% to 114.9%, suggesting the accuracy and feasibility of the method.

 Please wait while we load your content...
Please wait while we load your content...