Selectively detecting trace picric acid by reduced perylene bisimide with POSS substituents and their nanoaggregates†
Bo Yu,
Jiajun Ma,
Yujuan Zhang,
Gang Zou and
Qijin Zhang*
Key Laboratory of Soft Matter Chemistry, Key Laboratory of Optoelectronic Science and Technology, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui 230026, P. R. China. E-mail: zqjm@ustc.edu.cn
First published on 13th March 2015
Abstract
Reduced perylene bisimides (PBIs) with two substituents of polyhedral oligomeric silsesquioxane (POSS) are designed and synthesized for rapid and selective detection of picric acid in THF solution. Nanoaggregates of the reduced PBI show fluorescence that is also sensitive to the concentration change of picric acid.
Introduction
Picric acid (PA) has become a significant environmental pollutant because it has been widely used in the leather, pharmaceutical, and dye industries, as well as in the manufacture of explosives and rocket fuels, but it is also a strong irritant and allergen.1 Under these circumstances, many methods for detection of PA have been developed, in which fluorescence sensors show more advantages in terms of sensitivity, selectivity, simplicity and real-time for the detection of PA.2,3 The already existing materials for the fluorescence sensors include organic conjugated polymers4 or polymer composite film1 and metal–organic framework.5 All of them exhibit a fluorescent response to PA, likely through a host–guest interaction combined with an electron transfer process from the electron-rich fluorophore to the electron-deficient PA.2,6On the other hand, solid-state fluorescence sensing has received considerable attention.7,8 Effective solid aggregate fluorescence sensors based on organic materials are often demanding available organic substances that exhibit strong fluorescence in the solid state and suitable binding or absorption sites that can capture the analyte molecules.9 PBIs, for example, are often reported to detect aniline by modifying with substituent groups due to their strong π–π stacking and solid fluorescence.
PBIs have been extensively investigated as organic dyes due to their high molar absorptivity, high quantum yields of fluorescence with excellent photochemical and thermo-stability.10 Out of near-unity fluorescence quantum yields in solutions, as suitable luminophore in fluorescence sensors PBIs' derivatives are often designed and synthesized by two approaches: one is prolonging their main chains11–13 and the other is modifying their bay-substituents.14,15 Both of them are heavy workload and for the second method, the bay-substituted groups hinder the π–π stacking.
In this paper, a new strategy is proposed to extend sensor applications of PBIs, in which PBI is used as backbone and POSS as substituents to form organic–inorganic hybrid fluorescent molecules (PDP-1). The intramolecular organic–inorganic hybrid structure endows PBI with extraordinary properties such as excellent thermal stability and solubility, a low dielectric constant and an outstanding oxygen plasma etching resistance.16 Reduction of the carbonyl groups in PDP-1 is used to derive PDP-2 (shown in Fig. 1) with the strong electron-rich tertiary nitrogen centre, which has strong response to electron-deficient molecule such as PA and is prone to form hydrogen bonds with the hydroxyl of PA in aprotic solvents, resulting in much more interactions between PDP-2 and PA than other electron-deficient molecules.
Results and discussion
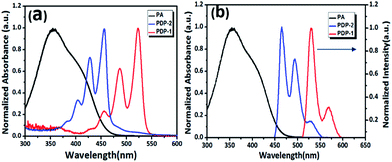
From UV-vis spectra of PDP-1 and PDP-2 in THF shown in Fig. 2a, it can be seen that the maximum absorption peaks of two samples are located at 525 nm and 460 nm, respectively. Compared with the absorption of PA (black line in Fig. 2a), it is easily found that the absorption band of PA has sensible overlap with the absorption of PDP-2, other than that of PDP-1, because there is a blue shift of 65 nm between two absorptions of PDP-1 and PDP-2. Under irradiation of light at 445 nm, PDP-2 has strong fluorescence at about 460 nm that is overlapped with the absorption of PA (Fig. 2b). From the results of the previous research for the detection of PA, it is known that the fluorescence quenching of PDP-2 might occur due to the possible formation of non-fluorescent complex when there is an interaction between PA containing strong electron-withdrawing groups and PDP-2 with strong electron-rich tertiary nitrogen.2 This expectation has been verified by experimental results shown in Fig. 3: by gradually adding PA solution of 18 μM to the solution of 1.5 μM PDP-2 in THF the fluorescence quenching is observed (the result has been corrected for the dilution introduced by the addition of the PA solution) (Fig. 3a). The mechanism about this fluorescence quenching is caused by interaction between PA and PDP-2 as shown in Fig. 3c, similar to the description in the reported work.17 Detailed information concerning this interaction can be obtained from the change of 1H NMR before and after adding trace PA, in which the resonant peak of the methylene linking with tert-nitrogen group is shifted downfield from 3.02 to 3.39 (Δσ = 0.37) (Fig. S4†) and the decrease in the fluorescence lifetime of PDP-2 in mixture solution (Fig. S5†) also imply there is energy transfer between PDP-2 and PA. Results shown in Fig. 3b have obviously testified that the quenching course agrees with the Stern–Volmer plot. The detection limit (3σ/slope) of PA is calculated at least down to 80 nM. In addition, the fluorescence quenching of PDP-2 with the addition of PA can also be observed by the naked eye (Fig. S6†), owing to the large Stern–Volmer data (Ksv = 1.19 × 106 L mol−1) comparing with other turn-off sensors (Table S1†).In order to investigate the PDP-2's property of recognition of nitro aromatic compounds, the fluorescence behaviour of PDP-2 toward different nitro compounds: picric acid (PA), 2,4,6-trinitrotoluene (TNT), 2,4-dinitrotoluene (DNT), 1,4-dinitrobenzene (DNB), p-nitro-toluene (NT), p-nitrobenzene (NB), p-toluylicacid (NTA), p-nitrobenzoic acid (NA), p-nitrophenol (NP), and benzoic acid (NBA), are performed with the same concentration (2 × 10−5 mol L−1). Hydrochloric acid (HCl) is also used as a blank sample to eliminate the influence of the proton because the tertiary nitrogen is easily to interact with protons.
The response of the fluorescence emission of PDP-2 in THF to PA, TNT, DNT, DNB, NT, NB, NTA, NP, NA, NBA and HCl is also well-established as shown in Fig. 3d, in which it is found that no significant change in PDP-2 fluorescence is observed by addition of equivalent amount of above nitro compounds, except PA (Fig. 3d). Because of a lack of tendency to protonate the tert-nitrogen group of PDP-2, nitro derivatives other than PA do not follow the ionic mechanism, which reduces their quenching efficiencies of the fluorescence of PDP-2.
Solid PDP-2 can be obtained by concentrating the solution of PDP-2 in THF, however, there is no fluorescence observed in this solid state PDP-2 (Fig. S7†). This behaviour is different from that of similar structure of PBI with β-cyclodextrin as substituents.9 In PDP-2, POSS substituent has a small volume that has no effect on the ordered arrangement of PDP-2, and fluorescence quenching would take place through energy transfer between ordered PDP-2 as shown in Fig. S8.†
In order to overcome this problem, nanoaggregates of PDP-2 were prepared by interfacial assembly.18 The morphology of the nanoaggregate has been observed by SEM as shown in Fig. 4b.
Comparing with the alkyl groups disubstituted PBIs, the fluorescence of the nanoaggregate of PDP-2 can be observed due to the unique stacking effect of POSS. Furthermore, under an excitation of light at 445 nm, the maximum is located at about 550 nm as shown in Fig. 4c, although the emission looks unlike that of PDP-2 in THF solution. The possible reason for this phenomenon is that large specific surface makes the ordered arrangement of PDP-2 loss, producing partly fluorescence quenching. In this way, it is expected that the tert-nitrogen group on the surface of nanoaggregates is still active to the PA molecules.
When a solution containing nanoaggregates of PDP-2 is dropped onto the paper, the photoluminescence also exists as shown in Fig. 4d. When the solution of PA is dropped onto the paper, the fluorescence quenching takes place as shown in Fig. 4d and e, in which it is found that the detected concentration of PA is 10−15 M in this way. This is a relatively low concentration for detecting PA in comparison with previous reported works.19
Conclusions
In summary, reduced PBI with two POSS substituents (PDP-2) has been synthesized for detection of PA. PDP-2 solution in THF shows a selective, sensitive, colorimetric, and ratiometric fluorescent quenching to PA with Ksv to be 1.19 × 106 L mol−1. The fluorescent quenching to PA is lost in solid state of PDP-2, however, nanoaggregates of PDP-2 prepared by interfacial assembly are found to have fluorescence that can be quenched by dropping PA solution in THF, and the lowest detection concentration is found to be 10−15 M. As a general design strategy, reduced PBI with POSS substituents may allow us to create further fluorescent sensor candidates for PA in the future.Acknowledgements
This work is supported by National Natural Science Foundation of China (no. 21074123, 91027024, 51173176 and 51273186).Notes and references
- G. He, H. Peng, T. Liu, M. Yang, Y. Zhang and Y. Fang, J. Mater. Chem., 2009, 19, 7347–7353 RSC.
- Y. Peng, A.-J. Zhang, M. Dong and Y.-W. Wang, Chem. Commun., 2011, 47, 4505–4507 RSC.
- Y. Ma, H. Li, S. Peng and L. Wang, Anal. Chem., 2012, 84, 8415–8421 CrossRef CAS PubMed.
- S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871–2883 RSC.
- K. S. Asha, K. Bhattacharyya and S. Mandal, J. Mater. Chem. C, 2014, 2, 10073–10081 RSC.
- N. Dey, S. K. Samanta and S. Bhattacharya, ACS Appl. Mater. Interfaces, 2013, 5, 8394–8400 CAS.
- V. Bellon-Maurel, O. Orliac and P. Christen, Process Biochem., 2003, 38, 881–896 CrossRef CAS.
- V. Bhalla, A. Gupta and M. Kumar, Org. Lett., 2012, 14, 3112–3115 CrossRef CAS PubMed.
- Y. Liu, K.-R. Wang, D.-S. Guo and B.-P. Jiang, Adv. Funct. Mater., 2009, 19, 2230–2235 CrossRef CAS.
- M. Franceschin, C. Bombelli, S. Borioni, G. Bozzuto, S. Eleuteri, G. Mancini, A. Molinari and A. Bianco, New J. Chem., 2013, 37, 2166–2173 RSC.
- N. Pasaogullari, H. Icil and M. Demuth, Dyes Pigm., 2006, 69, 118–127 CrossRef CAS PubMed.
- L. Zhong, F. Xing, W. Shi, L. Yan, L. Xie and S. Zhu, ACS Appl. Mater. Interfaces, 2013, 5, 3401–3407 CAS.
- A. Arulkashmir, B. Jain, J. C. John, K. Roy and K. Krishnamoorthy, Chem. Commun., 2014, 50, 326–328 RSC.
- A. J. Jimenez, M.-J. Lin, C. Burschka, J. Becker, V. Settels, B. Engels and F. Wurthner, Chem. Sci., 2014, 5, 608–619 RSC.
- H.-Y. Tsai, C.-W. Chang and K.-Y. Chen, Tetrahedron Lett., 2014, 55, 884–888 CrossRef CAS PubMed.
- L. Wang, Y. Ishida, R. Maeda, M. Tokita and T. Hayakawa, RSC Adv., 2014, 4, 34981–34986 RSC.
- V. Vij, V. Bhalla and M. Kumar, ACS Appl. Mater. Interfaces, 2013, 5, 5373–5380 CAS.
- J.-S. Hu, Y. G. Guo, H.-P. Liang, L.-J. Wan, C.-L. Bai and Y.-G. GuoWang, J. Phys. Chem. B, 2004, 108, 9734–9738 CrossRef CAS.
- S. Kaur, V. Bhalla, V. Vij and M. Kumar, J. Phys. Chem. C, 2014, 2, 3936–3941 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra16162a |
| This journal is © The Royal Society of Chemistry 2015 |