Simultaneous extraction of bisphenol A and tetrabromobisphenol A from milk by microwave-assisted ionic liquid microextraction†
Abstract
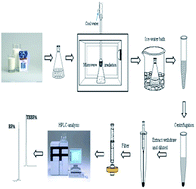
Considering bisphenol A (BPA) and tetrabromobisphenol-A (TBBPA) are ubiquitous environmental pollutants and widely manufactured into packaging materials, foodstuffs may be polluted by BPA and TBBPA. Additionally, there are very limited analytical methods for determining TBBPA in commercial milk samples. In this study, a rapid, simple and efficient method based on microwave-assisted ionic liquid microextraction (MAILME) combined with high-performance liquid chromatography (HPLC) was first applied to the simultaneous analysis of BPA and TBBPA in commercial milk samples. To achieve the optimum extraction performance, the experimental parameters of the MAILME, including type and amount of ionic liquid, microwave power, extraction time, pH and salt concentration in the sample, were evaluated and optimized. Under the optimum conditions, the detection limits were 0.01 and 0.02 μg L−1, and the quantification limits were 0.03 and 0.05 μg L−1 for BPA and TBBPA, respectively. The proposed method had good repeatability and reproducibility with RSDs lower than 5.6%. The recoveries of the analytes in the aged milk samples varied from 79.8 to 97.3%. The proposed method has been applied successfully to the determination of BPA and TBBPA in commercial milk samples with recoveries ranging from 78.2 to 99.8%. Compared with the other previous methods, the proposed method did not need to remove the fats and proteins in the milk, which made the sample pretreatment simple and rapid (5.0 min). And it also avoided the use of organic solvents. The proposed approach provides a new and promising alternative for simultaneously determining trace amounts of BPA and TBBPA in commercial milk samples or other similar samples.

 Please wait while we load your content...
Please wait while we load your content...