Interplay between the composition of LLDPE/PS blends and their compatibilization with polyethylene-graft-polystyrene in the foaming behaviour†
Guangchun Zhangab,
Yuanliang Wangab,
Haiping Xinga,
Jian Qiu*a,
Jiang Gongab,
Kun Yaoab,
Haiying Tanab,
Zhiwei Jianga and
Tao Tang*a
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China. E-mail: ttang@ciac.ac.cn; Fax: +86 431 85262827; Tel: +86 431 85262004
bGraduate School of the Chinese Academy of Sciences, Beijing 100039, China
First published on 9th March 2015
Abstract
Polyethylene-g-polystyrene (PE-g-PS) copolymers, which were prepared by the combination of the ROMP and ATRP method, were utilized to compatibilize LLDPE/PS blends. On one hand, the effect of PE-g-PS on the morphologies of LLDPE/PS blends was investigated. On the other hand, the influences of branch length and added amount of PE-g-PS on the cell morphology of foamed LLDPE/PS blends with different compositions were studied using supercritical CO2 as a physical foaming agent in a batch foaming process. It was found that the presence of PE-g-PS in the LLDPE/PS blends showed different influences on the foaming behaviour, strongly depending on the composition of the blends (i.e. the weight ratio of LLDPE and PS). How the interplay of compatibilization and composition of the LLDPE/PS blends affected the foaming behaviour of the LLDPE/PS blends was studied. A reasonable explanation was ascribed to consecutive states of the interfacial region, resulting from different phase structures of the blends. Compared to pristine LLDPE and PS, the blends with a sea-island phase structure showed the improved foam morphology, but the presence of PE-g-PS did not strongly influence the foaming behaviours of these blends. In contrast, the presence of PE-g-PS dramatically promoted the foaming ability of LLDPE/PS blends with a co-continuous phase structure. It was ascribed to the strengthened interfacial adhesion blocking the channel between two components through which CO2 was released, and the viscoelasticity of the blends was not the key factor to determine the foaming behaviour under the same foaming conditions in this work.
1. Introduction
Fabricating polymer alloys through mixing different polymer components is a powerful method to prepare a new material different from its parental components. Owing to poor compatibility between most different polymers, block or graft copolymers are often needed as compatibilizers in polymer blends. The compatibilization is important to stabilize the morphology and promote the properties of the resultant blends.1 Molecular structural parameters of block or graft copolymers play a key role in the compatibilization of polymer blends. Most previous research is mainly focused on the systems containing block copolymers as compatibilizers.2–8 However, when using a compatibilizer, the presence of block copolymers increases the melt viscosity of polymer blends due to its high molecular weight, so a higher shear rate was required when melt processing takes place.3,9,10 In contrast, graft copolymers with the same composition and total molecular weight displays a little increase in the melt viscosity at high shear frequency, although the melt viscosity of graft copolymers at lower shear frequency increases obviously.11–13 Now there have been many reports about the compatibilization of immiscible polymer blends,14–17 but few of them are focused on the effect of compatibilizers on foaming behaviour of polymer blends.Fabricating foamed materials from polymer blends are fascinating and challenging in application and scientific research field, because foamed polymer blends afford the potential of combining the advantageous properties of each component within the foamed material, and take advantage of the multiphase characteristics for the foaming process. Apart from the basic foaming conditions (temperature, pressure et al.), composition of the blends as well as other additives could affect the material properties (such as crystallinity,18 surface tension,19,20 melt strength,21–23 solubility and diffusivity of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24) and ultimately determine the foam morphology and properties.25–31 For example, Rachtanapun et al.32 have found that PP/HDPE = 30/70 blends, prepared by twin screw extruder at 100 rpm using CO2 as blowing agent, exhibit poor foam morphology. The authors thought that higher HDPE content caused the matrix to be too soft (low viscous), leading to cell coalescence. Increasing the content of PP (50/50 and 70/30 blends), the blend foam shows improved morphology, due to viscosity and stiffness appropriate for the development of a microcellular structure.33 The immiscible polymer blends show poor interface adhesion but pronounced surface activity, which favour cell nucleation. Rodrigue et al.34 employed three-dimensional analysis to study the interaction of foaming and blending in PP/HDPE blends during melt extrusion foaming using azodicarbonamide as blowing agent. Their results indicated that the foam morphologies of PP/HDPE blends were not substantially modified in the presence of compatibilizing agent (Kraton D 1102) except low dispersed phase concentration (10% and 90% PP). Zhai et al.35 found that the addition of PP-g-PS copolymers could improve the foaming properties of PP/PS (50/50) blends due to the improved interfacial compatibility. They provided a direct experimental proof for the heterogeneous nucleation theory, i.e. most cells were located at the compatibilied interface due to low energy barrier for cell nucleation.
24) and ultimately determine the foam morphology and properties.25–31 For example, Rachtanapun et al.32 have found that PP/HDPE = 30/70 blends, prepared by twin screw extruder at 100 rpm using CO2 as blowing agent, exhibit poor foam morphology. The authors thought that higher HDPE content caused the matrix to be too soft (low viscous), leading to cell coalescence. Increasing the content of PP (50/50 and 70/30 blends), the blend foam shows improved morphology, due to viscosity and stiffness appropriate for the development of a microcellular structure.33 The immiscible polymer blends show poor interface adhesion but pronounced surface activity, which favour cell nucleation. Rodrigue et al.34 employed three-dimensional analysis to study the interaction of foaming and blending in PP/HDPE blends during melt extrusion foaming using azodicarbonamide as blowing agent. Their results indicated that the foam morphologies of PP/HDPE blends were not substantially modified in the presence of compatibilizing agent (Kraton D 1102) except low dispersed phase concentration (10% and 90% PP). Zhai et al.35 found that the addition of PP-g-PS copolymers could improve the foaming properties of PP/PS (50/50) blends due to the improved interfacial compatibility. They provided a direct experimental proof for the heterogeneous nucleation theory, i.e. most cells were located at the compatibilied interface due to low energy barrier for cell nucleation.
As we know, PE and PS are two kinds of common resins, which are widely applied in the foamed materials. However, the strength and stiffness of PE foam are low, and the toughness of PS foam is poor. It is a simple method to compensate the shortcoming by fabricating polymer blend of these two components. In order to prepare high-performance PE/PS blend foam, it is necessary to improve the interfacial interaction between PE and PS by adding compatibilizers. To our knowledge, the studies about foaming behaviour of compatibilized PE/PS blend were rare in the scientific literature, only a US patent36 was related to this issue, in which the extrusion-foamed PE/PS blends using organic volatile blowing agent in the presence of hydrogenated styrene/butadiene block copolymers showed a good performance in the cushioning application. However, how the interplay of compatibilization and composition of PE/PS blends affects foaming behaviour of PE/PS blends is not studied.
In this work, PE-g-PS graft copolymers, which were prepared by the combination of ROMP and ATRP method (Scheme S1 in the ESI†), were utilized to compatibilize LLDPE/PS blends. Using supercritical CO2 as physical foaming agent in a batch foaming, the influences of branch length and added amount of PE-g-PS on the cell morphology of foamed LLDPE/PS blends with different composition were studied. We found that the presence of PE-g-PS in the LLDPE/PS blends showed different influence on the foaming behaviour, strongly depending on the composition of the blends (i.e. the weight ratio of LLDPE and PS). It was focused on how the interplay of compatibilization and composition of LLDPE/PS blends affected foaming behaviour of LLDPE/PS blends.
2. Experimental section
2.1. Materials
Linear low density polyethylene (LLDPE, DFDA-7042, MI = 2.0 g/10 min (ISO1133, 190 °C, 2.16 kg), Mw = 141 kg mol−1, PDI = 3.3) was supplied by Sinopec Maoming Company, China. Polystyrene (PS, Polyrex® PG-383, MI = 3.0 g/10 min (ISO1133, 200 °C, 5 kg), Mw = 372 kg mol−1, PDI = 1.7) was provided by Zhenjiang Chi Mei Chemical. Co., Ltd. Polyethylene-g-polystyrene (PE-g-PS) (used as a compatibilizer in this work) was synthesized through a method similar to the previous reports.37,38 The detail for the synthesis of PE-g-PS was provided in the ESI.†2.2. Preparation of LLDPE/PS blends
All blend samples were prepared by solution mixing method. The LLDPE with butylated hydroxytoluene (BHT) was first dissolved in refluxing toluene and kept under N2 atmosphere to prevent oxidation. The PE-g-PS and PS were subsequently added to the above hot solution. After the mixture formed a clear and homogeneous solution, the blend was precipitated into methanol, and dried under vacuum before melt pressed.2.3. Batch foaming
A stainless steel high-pressure vessel was used in batch foaming process. The high-pressure vessel was loaded with sample granules. After the high-pressure vessel was purged with low-pressure CO2, a given amount of CO2 was pumped into the vessel. The vessel was heated to predetermined foaming temperature and continuously charged with CO2 to the fixed pressure. The samples were saturated for 4 h to ensure equilibrium adsorption of CO2. Thereafter, the valve was rapidly opened to release the CO2. Then the high-pressure vessel was opened up, and the foamed samples were taken out for subsequent analyses.2.4. Characterization methods
The morphology of polymer blends was characterized by a XL 30 ESEM FEG scanning electron microscope (SEM). The compression-molded blend samples were cryofractured in liquid nitrogen. The fractured surfaces were coated with a thin layer of gold before SEM observation.The cell morphology of the foamed samples was characterized by an XL 30 ESEM FEG scanning electron microscope (SEM). The foamed samples were fractured in liquid nitrogen and SEM images of the fractured surfaces were taken. A representative micrograph containing 100 to 200 bubbles was obtained and the number of bubbles n in the micrograph was determined. The cell diameter was the average of the sizes of more than 100 cells on the SEM micrograph. The cell density (N0), the number of cells per cubic centimeter of solid polymer, was determined from eqn (1):
 | (1) |
Rheological measurements were performed on a rotation rheometer ARES G2 at 180 °C. The parallel plate with a diameter of 25 mm and a gap height of 0.8 mm was used. The test samples were first treated with 0.2 wt% Irganox B215 antioxidant and formed into disks with a diameter of 25 mm and a thickness of 1 mm by compression-molding at 180 °C and 10 MPa. Then, the samples were quenched at room temperature. The range of the frequency sweeps was from 0.05 to 200 rad s−1, and a strain of 1% was used, which was in the linear viscoelastic region for all samples. The rheometer oven was purged with dry nitrogen to avoid degradation of samples during measurements.
3. Results and discussion
3.1. The effect of PE-g-PS on the morphologies of LLDPE/PS blends
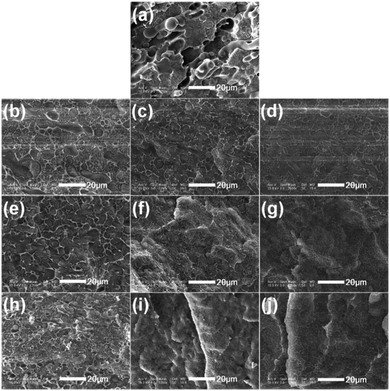
PE-g-PS graft copolymers with different branch length (Table 1) were utilized to compatibilize LLDPE/PS blends prepared by solution mixing. Fig. 1 illustrates SEM micrographs of cryogenic fractured surfaces of LLDPE/PS = 70/30 (by weight) blends with various amount of PE-g-PS. The dispersed PS particles and holes were evidently observed in the binary LLDPE/PS blend (Fig. 1a). This indicates poor interfacial adhesion between PS and LLDPE matrix. However, the morphology was remarkably different from the binary blend when an amount of PE-g-PS copolymers was added regardless of branch length (Fig. 1b–j). The LLDPE/PS/PE-g-PS blends showed flat fractured morphology, indicating enhanced interfacial adhesion between PS and LLDPE. Fig. 1e–g show the morphologies of the LLDPE/PS/PE-g-PS1.09k blend with the loading of PE-g-PS at 1.0, 2.5, 5.0 wt%, respectively. The average size of PS dispersed particles reduced with the concentration of the compatibilizer (Fig. S3a in the ESI†), which is in accordance with the prediction of the previous report.14 Furthermore, it can also be seen that the compatibilization of PE-g-PS promotes with the length of PS chains (Fig. S3b†).3.2. The effect of graft copolymers on foaming behaviours of LLDPE/PS = 70/30 blends
Fig. 2a and b show the cell morphologies of LLDPE and PS foams obtained using supercritical CO2 as foaming agent under a batch foaming process (13.3 MPa CO2 pressure at 113 °C). Both foams of LLDPE and PS exhibited large cell size (Dc) and small cell density (No) (Table 2). Fig. 2c shows the morphology of foamed LLDPE/PS = 70/30 blend. Very surprisingly, this binary LLDPE/PS blend was almost unfoamed (expansion ratio = 1.1). Furthermore, compared to the fractured surface morphology of unfoamed LLDPE/PS = 70/30 blend (Fig. 1a), the interfacial gap between two components in the foaming-processed blends became larger. Interestingly, the blends compatibilized by 1.0 wt% PE-g-PS0.34k exhibited fully foamed morphology (Fig. 2d), and the expansion ratio increased to 4.8. Compared to both LLDPE and PS, the cell diameter (Dc) of LLDPE/PS/PE-g-PS0.34k 70/30/1.0 decreased to ∼10 μm, and the cell density (No) increased to 4.7 × 109 cells per cm3 (Table 2). It is clear that the introduction of PE-g-PS0.34k can effectively improve the foaming behaviour of LLDPE/PS = 70/30 blend. The influence of PE-g-PS0.34k loading on the foaming behaviour of LLDPE/PS = 70/30 blend was also investigated. The increase of PE-g-PS0.34k content could further improve the foaming behaviour of LLDPE/PS/PE-g-PS0.34k (Fig. 2e–f). When the content of PE-g-PS0.34k increased from 1.0 to 5.0 wt%, the expansion ratio increased from 4.8 to 6.7, the cell Dc was about 9–10 μm, and the cell density No was increased to 8.8 × 109 cells per cm3. We also observed that, although PE-g-PS0.34k was added only 1.0 wt%, the overall foam structure was basically uniform (Fig. 2d). When the content of PE-g-PS0.34k increased to 5.0 wt%, the foam morphology became more uniform so we could not distinguish the foam districts from LLDPE and PS (Fig. 2f).| Sample | Dda (μm) | Expansion ratio | Dcb (μm) | Noc (109 cells cm−3) | |
|---|---|---|---|---|---|
| a Mean droplet diameter.b Average cell diameter.c Cell density. | |||||
| LLDPE | — | 2.7 | 18.7 | 0.286 | |
| PS | — | 5.6 | 50.9 | 0.058 | |
| LLDPE/PS 70/30 | 14.7 | 1.1 | — | — | |
| LLDPE/PS/PE-g-PS0.34k | 70/30/1.0 | 4.84 | 4.8 | 10.5 | 4.69 |
| 70/30/2.5 | 4.70 | 5.5 | 9.6 | 6.91 | |
| 70/30/5.0 | 4.06 | 6.7 | 10.1 | 8.81 | |
Fig. 3 exhibits the SEM micrographs of foamed LLDPE/PS (70/30) blends (under the conditions: T = 110 °C and P = 13.3 MPa) compatibilized by PE-g-PS0.34k, PE-g-PS1.09k and PE-g-PS1.59k, respectively. Here the foaming temperature (T = 110 °C) was lower than the previous one (T = 113 °C), but the pressure kept the same. From Fig. 3a–c, it could also be seen that increasing the content of PE-g-PS0.34k resulted in an improved foaming behaviour (the cell parameters were summarized in Table 3) even though the foaming conditions were changed. Comparing the foaming results at different foaming temperatures (Table 2 vs. 3), it was found that the cell density increased and the cell size decreased at a lower foaming temperature. Decreasing the temperature will increase the viscosity of the substrate material, causing the force restricting cell growth to increase and the diffusivity of CO2 within the substrate to decrease.39,40 If the CO2 diffusivity decreases, the sorption of CO2 during CO2 dissolution decreases, thus, the quantity of CO2 in the sample is lower than samples treated at higher temperatures.41 These factors lead to decreased cell size. Decreased temperature results in a higher degree of swelling by CO2 and thus the formation of more nuclei, which increases cell density.39,40 The cell density of compatibilized blends was improved with the increase of the content of PE-g-PS0.34k or PE-g-PS1.09k. However, when the content of PE-g-PS1.59k increased from 1.0 wt% to 5.0 wt%, the cell density slightly changed. There are following possible reasons for weak dependence of the cell density on the content of PE-g-PS1.59k. On one hand, the cell density of the blend sample containing 1.0 wt% PE-g-PS1.59k is much higher than those of the counterparts containing PE-g-PS0.34k or PE-g-PS1.09k, which results from the better compatibilization of PE-g-PS1.59k due to the presence of long PS branches. On the other hand, the addition of more PE-g-PS1.59k can reduce the average droplet diameter (Table 3), so the heterogeneous nucleation sites provided by the interfaces between the dispersed PS droplets and the LLDPE matrix should increase. Theoretically speaking, the cell density of the blends will increase. Thus, the possible reason is the reduction of the nucleation efficiency during foaming process in the case containing 5 wt% PE-g-PS1.59k, which results in the reduction of cell density.42 In summary, the compatibilized LLDPE/PS (70/30) blends exhibited improved foaming ability (such as higher expansion ratio, increased cell density and reduced cell size) compared to pure LLDPE, PS and the corresponding binary blend.
| Sample | Dda (μm) | Expansion ratio | Dcb (μm) | Noc (109 cells cm−3) | |
|---|---|---|---|---|---|
| a Mean droplet diameter.b Average cell diameter.c Cell density. | |||||
| PE/PS 70/30 | 14.7 | 1.1 | — | — | |
| LLDPE/PS/PE-g-PS0.34k | 70/30/1.0 | 4.84 | 2.7 | 6.9 | 4.56 |
| 70/30/2.5 | 4.70 | 4.7 | 7.5 | 9.20 | |
| 70/30/5.0 | 4.06 | 5.3 | 7.8 | 11.2 | |
| LLDPE/PS/PE-g-PS1.09k | 70/30/1.0 | 4.57 | 5.8 | 10.7 | 5.60 |
| 70/30/2.5 | 3.47 | 5.2 | 9.3 | 7.78 | |
| 70/30/5.0 | 1.75 | 5.5 | 8.1 | 11.0 | |
| LLDPE/PS/PE-g-PS1.59k | 70/30/1.0 | 3.66 | 5.2 | 8.8 | 8.44 |
| 70/30/2.5 | 2.40 | 4.4 | 7.8 | 9.76 | |
| 70/30/5.0 | 1.74 | 5.6 | 9.1 | 9.00 | |
3.3. The effect of blend composition on foaming behaviours of uncompatibilized and compatibilized LLDPE/PS blends
The influence of PE-g-PS on the foaming behaviours of blends with different compositions was further investigated, mainly via changing the weight ratio of LLDPE/PS from 90/10 to 50/50 while keeping the content of PE-g-PS0.34k (1.0 wt%). Fig. 4 shows the influence of PE-g-PS on the morphology of LLDPE/PS blends with various compositions before foaming processing. The samples were etched by THF to remove PS component before SEM observation. It can be seen that the size of dispersed PS particles does not reduce remarkably in the cases of LLDPE/PS = 90/10 and 80/20 in the presence of PE-g-PS. When the content of PS increased to 40 and 50 wt%, the blends exhibited co-continuous phase structure. In these cases, the addition of PE-g-PS dramatically improved the dispersion states of both components.Fig. 5 shows foam morphologies of the above blends. As shown in Fig. 5a, the LLDPE/PS = 90/10 binary blend showed fully foamed morphology. Compared to pure LLDPE, the expansion ratio increased to 8.1, the cell size decreased to 14.4 μm, and the cell density increased to 3.03 × 109 cells per cm3 (Table 4). However, the addition of PE-g-PS0.34k in this binary blend did not remarkably improve the foaming behaviour (Fig. 5e, and cell parameters in Table 4) comparing with the foam morphology of the pure binary blend. A similar changing trend was observed in LLDPE/PS = 80/20 blend after adding 1.0 wt% PE-g-PS0.34k (Fig. 5b and f, and Table 4). Obviously, blending can lead to a significant increase in cell density compared to pure LLDPE, indicating that the cell nucleation is strongly influenced by the dispersion state of PS phase and the morphology of the blend. The addition of a small amount of PS (10 to 20 wt%) significantly increases the cell density, resulting from a heterogeneous nucleation effect of dispersed PS phase, which dominates over the homogeneous nucleation under the foaming conditions. The addition of graft copolymers did not change the average size of PS droplets so much (Fig. 4e and f), indicating that the heterogeneous nucleation sites provided by the interfaces between the dispersed PS droplets and the LLDPE matrix kept almost constant. As a result, the cell density did not change so much in the presence of graft copolymer.
| Sample | Expansion ratio | Dc (μm) | No (109 cells cm−3) |
|---|---|---|---|
| LLDPE | 2.7 | 18.7 | 0.286 |
| PE/PS 90/10 | 8.1 | 14.4 | 3.03 |
| PE/PS/PE-g-PS0.34k 90/10/1.0 | 8.7 | 13.9 | 3.66 |
| PE/PS 80/20 | 6.2 | 13.4 | 3.03 |
| PE/PS/PE-g-PS0.34k 80/20/1.0 | 6.5 | 13.4 | 3.22 |
| PE/PS 70/30 | 1.1 | — | — |
| PE/PS/PE-g-PS0.34k 70/30/1.0 | 4.8 | 10.5 | 4.69 |
| PE/PS 60/40 | 1.4 | — | — |
| PE/PS/PE-g-PS0.34k 60/40/1.0 | 3.4 | 7.1 | 5.27 |
| PE/PS 50/50 | 1.4 | — | — |
| PE/PS/PE-g-PS0.34k 50/50/1.0 | 3.6 | 7.3 | 5.79 |
| PE/PS 40/60 | 1.2 | — | — |
| PE/PS/PE-g-PS1.59k 40/60/1.0 | 5.4 | 10.1 | 6.58 |
| PE/PS 30/70 | 1.4 | — | — |
| PE/PS/PE-g-PS1.59k 30/70/1.0 | 5.4 | 8.0 | 9.79 |
| PE/PS 20/80 | 5.5 | 9.9 | 5.36 |
| PE/PS/PE-g-PS1.59k 20/80/1.0 | 6.2 | 8.8 | 8.72 |
| PE/PS 10/90 | 5.8 | 9.4 | 5.37 |
| PE/PS/PE-g-PS1.59k 10/90/1.0 | 4.7 | 8.5 | 7.05 |
| PS | 5.6 | 50.9 | 0.058 |
Similar to the case of LLDPE/PS 70/30 binary blend, the LLDPE/PS 60/40 binary blend did not form the foam structure as well (Fig. 5c). The addition of 1.0 wt% PE-g-PS0.34k dramatically improved the foaming behaviour of LLDPE/PS 60/40 blend (Fig. 5g), which is similar to the aforementioned cases of LLDPE/PS/PE-g-PS0.34k 70/30/1.0 vs. LLDPE/PS 70/30 (Fig. 2c and d). From Table 4, the average cell size was 7.1 μm in LLDPE/PS/PE-g-PS0.34k 60/40/1.0, the cell density was 5.27 × 109 cells per cm3. It was of interest that the binary LLDPE/PS = 50/50 blend displayed distinct fractured morphology (Fig. 5d), in which the foamed regions belonged to the PS phase, and the unfoamed regions came from the LLDPE phase (from the etched foam morphology in Fig. 6). Similarly, when 1.0 wt% PE-g-PS0.34k was added, the compatibilized blends exhibited fully foamed morphology. In a word, the presence of PE-g-PS could dramatically improve foaming capacity of LLDPE/PS blends with co-continuous phase structure. The foaming results of LLDPE/PS blends with the composition from 40/60 to 10/90 with PE-g-PS1.59k also supported the above conclusion (Fig. S4 and S5 in the ESI†). The observed phenomenon in this work is different from the previous reports.34 This difference might result from the different polymer blends or different foaming processes, which need to be more studied.
 | ||
| Fig. 6 SEM micrographs for cryogenic fractured LLDPE/PS (50/50) after foaming process (a) and further etched surface by THF (b). | ||
In order to further study the foaming behaviour of LLDPE/PS blends with various compositions before and after compatibilized by PE-g-PS, we decreased the foaming temperature to 100 °C, and kept CO2 pressure (13.3 MPa) and saturation time constant. The influence of foaming temperature on the foam morphology is quite complex since it influences the gas solubility, nucleation rate as well as polymer viscosity. From Fig. 7, it can be seen that LLDPE did not foam and PS could foam expectedly under this foaming condition. When a small amount of PS was added into the LLDPE (10–20 wt%), the foaming capacity of LLDPE/PS blends was significantly improved (Fig. 7c and e). This indicates that the dispersed PS particles in LLDPE matrix act as nucleation agents for the foaming process of LLDPE. In this case, the addition of PE-g-PS can further increase the cell density. However, when the content of PS was 30–50 wt%, the binary LLDPE/PS blends presented very poor foaming behaviour. There were a lot of gaps in the interfacial region between two components (Fig. 7g, i and k). These gaps were larger than those of unfoamed samples. This means that a lot of CO2 was released through the interfacial region.
 | ||
| Fig. 7 SEM images for the foamed LLDPE/PS blends with various composition before and after compatibilized by PE-g-PS (the foaming temperature: 100 °C, and kept CO2 pressure and saturation time the same as the foaming conditions shown in Fig. 2 and 5). | ||
3.4. Insight into the influence of PE-g-PS on foaming behaviours of LLDPE/PS blends with different composition
According to the above results, it can be seen that the compatibilization of PE-g-PS play a different role in the foam morphologies of LLDPE/PS with various compositions. What is the mechanism behind the foaming behaviour of compatibilized LLDPE/PS blends with different composition? It is well known that the cell morphology is influenced by the nucleation of bubbles and their growth during the foaming process. Compared to single polymer, there are more factors influencing the foaming process of polymer blends, not only including gas solubility in the material, the rate of gas loss (diffusivity) and rheological properties of polymer materials, but also multiphase structure and interfacial region between different components. The CO2 gas uptake in the blend materials depends on the composition (and crystallinity in the blends containing crystalline component) of blends under the fixed foaming processing conditions. The presence of a small amount of PE-g-PS does not obviously change the weight ratio of LLDPE to PS. The influence of added PE-g-PS on crystalline properties (such as crystallinity and crystal form) of PE component in the blends was investigated by means of DSC and WAXD measurements (Fig. S6 and S7†). The results showed that the addition of PE-g-PS did not show an obvious effect on the crystallinity and crystal form of LLDPE component in the blends. Interestingly, compared to the unfoamed samples, all the foamed samples presented double melting peaks and a slightly high crystallinity of LLDPE component in the blends (the crystallinities for unfoamed LLDPE/PS = 60/40 and foamed LLDPE/PS = 60/40 were 36.8% and 39.5%, respectively) despite the presence of PE-g-PS. Moreover the crystallinity of LLDPE component in the foamed binary blends and compatibilized blends were very close (the crystallinities of 39.5% and 38.3% for foamed LLDPE/PS = 60/40 and foamed LLDPE/PS/PE-g-PS0.34k = 60/40/1.0, respectively, calculated by DSC). Thus the crystalline properties of the blends are not a key factor to the obvious difference of foaming behaviour between the binary blends and the compatibilized blends. Other factors, such as rheological properties, phase morphology and interfacial adhesion, might determine the difference in foaming behaviour and cell morphologies of LLDPE/PS binary blend and compatibilized LLDPE/PS blend, especially the last two factors among which affects the diffusion process of CO2. In this work, the rheological measurements showed that the complex viscosity (η*) and storage modulus (G′) of LLDPE/PS (70/30) was higher than pure LLDPE and lower than PS (Fig. S8 in the ESI†). This indicated that the viscoelasticity of the binary blends was in between LLDPE and PS. Because both LLDPE and PS can be foamed under the fixed foaming conditions, naturally it is expected that the binary blends should be foamed under the same foaming conditions. However, the foaming result indicated that the foaming behaviour of the binary LLDPE/PS (70/30) blend was very poor. Furthermore, although the complex viscosity (η*) and storage modulus (G′) of LLDPE/PS/PE-g-PS0.34k = 70/30/1.0 was almost the same as LLDPE/PS = 70/30 (Fig. S8†), the foam morphologies of both the samples were totally different. These results demonstrated that the viscoelasticity of the samples is not the key factor to determine the foaming behaviour among these samples under the same foaming conditions.It is well known that various phase structures can be developed when two immiscible polymers are mixed together to make polymer blends, depending on the composition and viscosity ratio of two components. Particularly, in the case of LLDPE/PS blends, the phase morphology will change from sea-island to co-continuous phase structure and to sea-island phase structure again with increasing the content of PS. Whether the interfacial region is consecutive or not is related to the phase structure of LLDPE/PS blends. The interfacial region is isolated in the blends with sea-island phase structure. Otherwise, the interfacial region is consecutive in the blends with co-continuous phase structure (Fig. S9†). Generally, the presence of void fraction has a strong effect on the diffusivity more than the solubility of gas in the polymer materials.27 Zhai et al. have found that the diffusion coefficients of CO2 for all PP/PS (50/50) blends are higher than those of the two pure polymers.35 It is believed that the diffusion coefficients of CO2 in LLDPE/PS = 70/30 blend with irregular dispersed morphology (not spherical dispersed morphology) or LLDPE/PS = 50/50 blend (co-continuous phase structure) will also increase relative to the LLDPE or PS. According to the classical nucleation theory, CO2 tend to assemble at the interface due to lower energy barrier, once depressurizing the consecutive interfacial region provided channels through which the assembled CO2 diffuse from the bulk to the environment rapidly and no CO2 left for cell growth. The enlarged gaps between two components in the foamed LLDPE/PS = 70/30 (or 50/50) blend provided the evidence of CO2 escaping from the interfacial region. As a result, LLDPE/PS blends with the composition of 70/30–30/70 could not efficiently form cell structures during the foaming process.
In contrast, when the composition of LLDPE/PS blends was 90/10, 80/20, 20/80 or 10/90, the blends showed a typical of sea-island phase structure (Fig. 4a and b, and S4c and S4d†), that is, spherical dispersed phase was distributed in the matrix, therefore, the interfacial regions are isolated from each other in the blends. In this case, the enriched foaming gas in the interfacial region could not be released rapidly to the environment due to the presence of the continuous phase outside the interfacial region. So the foam structure could be formed under the same foaming conditions. Fig. S10 in the ESI† showed that there were unfoamed particles (LLDPE particles) left in the foamed LLDPE/PS 20/80 blends after etching the sample by THF at room temperature. This indicated that the foamed structure was composed of PS component (continuous phase), and LLDPE as dispersed phase was not foamed. It is well known that the poorly bonded interfacial regions of immiscible polymer blends have lower activation energy for bubble nucleation.26 This means that the interface in the immiscible blends could be favourable to the formation of nucleating sites for bubble growth. Thus the presence of a small amount of PS in LLDPE matrix (such as LLDPE/PS = 90/10 or 80/20 blends) results in obviously increased cell density and expansion ratio compared to pristine LLDPE.
When the graft copolymer is added, the interfacial adhesion between two components will be strengthened. In the case of LLDPE/PS blends with co-continuous phase structure, the channel provided by interfacial region for the diffusion of dissolved CO2 into the environment was blocked. Meanwhile, the enlarged surface areas and lower energy barrier were favourable for the bubble nucleation and growth of cell. Consequently, the introduction of 1.0 wt% PE-g-PS dramatically improved foaming behaviour of LLDPE/PS blends (such as LLDPE/PS = 70/30 and 50/50 blends). In addition, as shown in Fig. 1, the average size of the dispersed phase decreased with the content of PE-g-PS (also see Fig. S3a†). The increased interfacial areas promoted heterogeneous nucleation, resulting in the formation of more nuclei for foaming. Therefore, the cell density of foamed LLDPE/PS blends was dramatically increased with the content of PE-g-PS. Comparing the foam morphologies of LLDPE/PS/PE-g-PS blends (70/30/1.0–50/50/1.0) before with after etching by THF, it was found that a part of frame structure of the foams was uniformly etched by THF (Fig. S11 in the ESI†). This indicated that the foam structure was composed of both LLDPE and PS, and it was difficult to distinguish the foam regions from LLDPE and PS. In contrast, the presence of PE-g-PS in LLDPE/PS blends with sea-island phase structure did not strongly change the foaming behaviour of LLDPE/PS blends, although the interfacial adhesion was improved. The phenomenon was ascribed to the interfacial region which was isolated in the blends with sea-island phase structure. In this case, whether adding compatibilizer or not will not influence the diffusion of CO2 in the blend bulk.
Another interesting phenomenon observed in this work is that PS component formed foam structure in the binary LLDPE/PS = 50/50 blend after foaming process. Poor interfacial adhesion between two components in LLDPE/PS = 50/50 blend with co-continuous structure was very clear. Although a large amount of dissolved CO2 escaped rapidly to the environment through the interface (large gap was also observed), the CO2 dissolved in PS matrix could still nucleate and the cell grew. Very recently, the latest report has studied the unique microcellular skin-core structures embedded in PLA/PS foams.43 It is thought that this interesting phenomenon of confined foaming behavior is involved in several factors, such as the weight ratio of components and phase structures (the shape and size of interface) of polymer blends, etc.
4. Conclusions
The foaming behaviour of LLDPE/PS blends with or without PE-g-PS as compatibilizer was studied by batch foaming. The compatibilization of PE-g-PS and the blend composition strongly affected the foaming behaviour of LLDPE/PS blends. When the content of one component (LLDPE or PS) was lower than 20 wt%, the morphology of LLDPE/PS blends presented sea-island structure. In this case, the blends showed improved foaming behaviours (i.e. decreased cell size and increased cell density) comparing with pure LLDPE and PS, but the addition of PE-g-PS did not further change the foam morphology of these blends. When the composition of LLDPE/PS blends was 70/30–30/70 by weight, the blends presented co-continuous phase structure. In this case, the binary blends showed very poor foaming ability. With the addition of PE-g-PS, the blends exhibited well foamed morphology, indicating dramatical improvement of foamability of these blends. This phenomenon did not show close relation with the change in melt viscosity of LLDPE/PS blends after adding the compatibilizer. The reasonable explanation was ascribed to consecutive state of interfacial region, resulting from different phase structure of the blends. The interfacial region is isolated in the blends with sea-island phase structure (LLDPE/PS blends with the composition of 90/10, 80/20, 20/80 and 10/90). Otherwise, the interfacial region is consecutive in the blends with co-continuous phase structure (LLDPE/PS blends with the composition of 70/30–30/70). In the case of LLDPE/PS blends with co-continuous phase structure, as the consecutive interfacial region between two components (due to the poor interfacial adhesion) provided channels through which gas could rapidly diffuse from the blend bulk to the environment, the binary blends with co-continuous phase structure showed a poor foaming capability. The presence of PE-g-PS blocked the channel provided by interfacial region for the diffusion of dissolved CO2 into the environment. However, the foam morphologies of the compatibilized blends by PE-g-PS with different PS length did not show obvious difference under the present foaming conditions. It is of interest that three kinds of foamed materials can be prepared from LLDPE/PS blends under the batching foaming process, depending on the blend composition and the compatibilization. Only the continouns component formed the foamed structure and the dispersed component acted as nucleating agent in the blends with sea-island phase structure. In contrast, both LLDPE and PS components in the blends with co-continuous phase structure were foamed in the comaptibilized blends, but only PS component was foamed in the binary blends (such as LLDPE/PS = 50/50).Acknowledgements
The financial supports from National Natural Science Foundation of China (51233005 and 21304089) and the Ministry of Science and Technology of China (2015AA033901) are greatly appreciated.References
- D. Gersappe, D. Irvine, A. C. Balazs, Y. Liu, J. Sokolov, M. Rafailovich, S. Schwarz and D. G. Peiffer, Science, 1994, 265, 1072 CAS.
- G. Basseri, M. Mehrabi Mazidi, F. Hosseini and M. K. Razavi Aghjeh, Polym. Bull., 2014, 71, 465 CrossRef CAS PubMed.
- E. Van Hemelrijck, P. Van Puyvelde, C. W. Macosko and P. Moldenaers, J. Rheol., 2005, 49, 783 CrossRef CAS.
- S. Lyu, T. D. Jones, F. S. Bates and C. W. Macosko, Macromolecules, 2002, 35, 7845 CrossRef CAS.
- P. Van Puyvelde, S. Velankar and P. Moldenaers, Curr. Opin. Colloid Interface Sci., 2001, 6, 457 CrossRef CAS.
- M. Heino, J. Kirjava, P. Hietaoja and J. A. Seppälä, J. Appl. Polym. Sci., 1997, 65, 241 CrossRef CAS.
- C. W. Macosko, P. Guégan, A. K. Khandpur, A. Nakayama, P. Marechal and T. Inoue, Macromolecules, 1996, 29, 5590 CrossRef CAS.
- U. Sundararaj and C. W. Macosko, Macromolecules, 1995, 28, 2647 CrossRef CAS.
- A. Chirawithayaboon and S. Kiatkamjornwong, J. Appl. Polym. Sci., 2004, 91, 742 CrossRef CAS.
- I. Fortelný, J. Mikešová, J. Hromádková, V. Hašová and Z. Horák, J. Appl. Polym. Sci., 2003, 90, 2303 CrossRef.
- P. Charoensirisomboon, T. Chiba, S. I. Solomko, T. Inoue and M. Weber, Polymer, 1999, 40, 6803 CrossRef CAS.
- N. Kitayama, H. Keskkula and D. R. Paul, Polymer, 2000, 41, 8041 CrossRef CAS.
- J. K. Kim and H. Lee, Polymer, 1996, 37, 305 CrossRef CAS.
- T. Tang and B. Huang, Polymer, 1994, 35, 281 CrossRef CAS.
- T. Tang, Z. Lei and B. Huang, Polymer, 1996, 37, 3219 CrossRef CAS.
- L. Wang, H. Tan, J. Gong and T. Tang, J. Appl. Polym. Sci., 2014, 131, 40126 Search PubMed.
- T. Tang, Z. Lei, X. Zhang, H. Chen and B. Huang, Polymer, 1995, 36, 5061 CrossRef CAS.
- S. Doroudiani, C. B. Park and M. T. Kortschot, Polym. Eng. Sci., 1998, 38, 1205 Search PubMed.
- H. Li, L. J. Lee and D. L. Tomasko, Ind. Eng. Chem. Res., 2003, 43, 509 CrossRef.
- L. M. Matuana, C. B. Park and J. J. Balatinecz, Polym. Eng. Sci., 1997, 37, 1137 CAS.
- T. Mizumoto, N. Sugimura, M. Moritani, Y. Sato and H. Masuoka, Macromolecules, 2000, 33, 6757 CrossRef CAS.
- P. Zhang, N. Q. Zhou, Q. F. Wu, M. Y. Wang and X. F. Peng, J. Appl. Polym. Sci., 2007, 104, 4149 CrossRef CAS.
- K. Yao, H. Tan, Y. Lin, G. Zhang, J. Gong, J. Qiu, T. Tang, H. Na and Z. Jiang, RSC Adv., 2014, 4, 64053 RSC.
- X. Han, J. Shen, H. Huang, D. L. Tomasko and L. J. Lee, Polym. Eng. Sci., 2007, 47, 103 CAS.
- D. L. Tomasko, A. Burley, L. Feng, S.-K. Yeh, K. Miyazono, S. Nirmal-Kumar, I. Kusaka and K. Koelling, J. Supercrit. Fluids, 2009, 47, 493 CrossRef CAS PubMed.
- J. S. Colton and N. P. Suh, Polym. Eng. Sci., 1987, 27, 493 CAS.
- P. Rachtanapun, S. E. M. Selke and L. M. Matuana, J. Appl. Polym. Sci., 2003, 88, 2842 CrossRef CAS.
- C. Zepeda Sahagún, R. González Núñez and D. Rodrigue, J. Appl. Polym. Sci., 2007, 106, 1215 CrossRef.
- T. A. Walker, D. J. Frankowski and R. J. Spontak, Adv. Mater., 2008, 20, 879 CrossRef CAS.
- Q. Wu, C. B. Park, N. Zhou and W. Zhu, J. Cell. Plast., 2009, 45, 303 CrossRef CAS PubMed.
- H. Ruckdäschel, P. Gutmann, V. Altstädt, H. Schmalz and A. E. Müller, Adv. Polym. Sci., 2010, 227, 199 CrossRef.
- P. Rachtanapun, S. E. M. Selke and L. M. Matuana, Polym. Eng. Sci., 2004, 44, 1551 CAS.
- P. Rachtanapun, S. E. M. Selke and L. M. Matuana, J. Appl. Polym. Sci., 2004, 93, 364 CrossRef CAS.
- C. Z. Sahagún, R. González-Núñez and D. Rodrigue, J. Cell. Plast., 2006, 42, 469 CrossRef PubMed.
- W. Zhai, H. Wang, J. Yu, J. Dong and J. He, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 1641 CrossRef CAS.
- N. Takeda, US Pat., 4 847 150, 1989.
- Y. Xu, C. M. Thurber, T. P. Lodge and M. A. Hillmyer, Macromolecules, 2012, 45, 9604 CrossRef CAS.
- M. A. Hillmyer, W. R. Laredo and R. H. Grubbs, Macromolecules, 1995, 28, 6311 CrossRef CAS.
- K. A. Arora, A. J. Lesser and T. J. McCarthy, Macromolecules, 1998, 31, 4614 CrossRef CAS.
- S. K. Goel and E. J. Beckman, Polym. Eng. Sci., 1994, 34, 1137 CAS.
- G. Gedler, M. Antunes and J. I. Velasco, J. Supercrit. Fluids, 2014, 88, 66 CrossRef CAS PubMed.
- K. Goren, L. Chen, L. S. Schadler and R. Ozisik, J. Supercrit. Fluids, 2010, 51, 420 CrossRef CAS PubMed.
- X. Liao, H. Zhang, Y. Wang, L. Wu and G. Li, RSC Adv., 2014, 4, 45109 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The details for the synthesis and characterization of PE-g-PS, the change trend of dispersed phase, morphologies of LLDPE/PS blends before and after foaming, and the plot of complex viscosity and storage modulus vs. angular frequency. See DOI: 10.1039/c4ra16084c |
| This journal is © The Royal Society of Chemistry 2015 |