Graphene/cotton composite fabrics as flexible electrode materials for electrochemical capacitors
Abstract
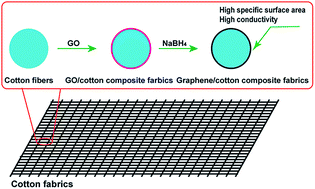
Graphene/cotton composite fabrics were successfully synthesized via a facile “dipping and drying” process followed by a NaBH4 reduction method. The flexible 3D conductive network constructed by graphene sheets greatly enhances the conductivity of cotton fabrics. The morphology, structure and conductivity of the graphene/cotton composite fabrics were detailed characterized using scanning electron microscopy (SEM), fourier transform infrared spectroscopy (FTIR) and a standard four-point probe method. Electrochemical measurements demonstrated that the graphene/cotton composite fabrics were used as flexible electrodes for electrochemical capacitors showing good capacitance, excellent mechanical flexibility, and good stability, which may lead to their future potential application in flexible and wearable electronic devices.

 Please wait while we load your content...
Please wait while we load your content...