Synthesis of Ag@SiO2 yolk–shell nanoparticles for hydrogen peroxide detection†
Abstract
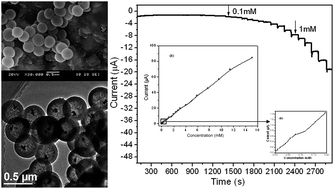
Yolk–shell nanostructures are a potential platform for the application of sensors and detection. In this paper, Ag@SiO2 yolk–shell nanoparticles (YSNs) were synthesized by a facile “two solvents” impregnation–reduction approach. XRD, SEM, TEM and N2 adsorption characterization results revealed that the resultant Ag@SiO2 YSNs possess distinctive structures, such as movable cores, perpendicular mesoporous channels, protective shells and hollow cavities. A nonenzymatic H2O2 sensor was constructed using Ag@SiO2 YSNs as sensing interface. A three-electrode system was used for the measurement. Electrochemical results indicate that the Ag@SiO2 YSNs modified electrode exhibits outstanding performance toward the H2O2 reduction, with a faster amperometric response, a lower detection limit (3.5 μM) and a wider linear range (0.1–15 mM) than that based on Ag@SiO2 composites, which was synthesized by a direct impregnation method.

 Please wait while we load your content...
Please wait while we load your content...