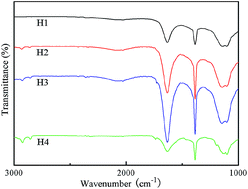
Carbon fabric was treated by HNO3 under hydrothermal conditions. The oxygenated functional groups were supported by Fourier transform infrared-attenuated total reflectance spectroscopy. The hydrophilicity was supported by the contact angle instrument. The treated carbon fabric was then used to prepare carbon fabric/resin friction materials. The friction and wear behaviors of the carbon fabric/resin friction material were evaluated by a friction tester. Experimental results showed that the carbon fabric treated by hydrothermal oxidation led to changes in surface morphology of fibers and appearance of carbonyl groups, which effectively improved the bonding strength of carbon fabric and resin. Moreover, hydrothermal oxidation treatment from 100 °C to 120 °C was proved beneficial to tribological properties.