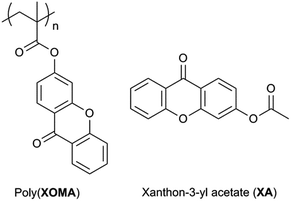
A high triplet-energy polymer: synthesis and photo-physical properties of a π-stacked vinyl polymer having a xanthone moiety in the side chain†
Hiroyoshi Sugino,
Yasuhito Koyama and
Tamaki Nakano*
Catalysis Research Center (CRC), Hokkaido University, N 21, W 10, Kita-ku, Sapporo 001-0021, Japan. E-mail: tamaki.nakano@cat.hokudai.ac.jp; Fax: +81-11-7069156; Tel: +81-11-7069155
First published on 18th February 2015
Abstract
Poly(xanthon-3-yl methacrylate) (poly(XOMA)) and poly(xanthon-3-yl methacrylate-co-methyl methacrylate) (poly(XOMA-co-MMA)) having a π-stacked conformation were synthesized as host material candidates for phosphorescence-based light emitting diodes. Poly(XOMA) harvested photo excitation energy for blue phosphorescent emission of iridium bis[(4,6-difluorophenyl)pyridinato-N,C2]picolinate (FIrpic) in a CHCl3 solution and in film.
Phosphorescence-emitting diodes (LEDs) have been recognized to be more important than fluorescent ones1–6 because the internal fluorescence quantum yield (Φ) is at a maximum around 25% (ref. 7) and the quantum yield of phosphorescent emitting materials can reach almost 100%. In constructing a phosphorescent OLED device, development of a polymeric host material dispersing emitting compounds is indispensable. Energy transfer may occur from a triplet excited host to an emitter to give a triplet excited state. It is also possible that a singlet-excited host excites a triplet emitter to lead to a singlet excited state and a triplet-excited emitter is formed through intersystem crossing. In either case, a good host material has to have a higher lowest triplet (T1) energy level compared with that of a phosphorescent emitter and efficient singlet or triplet energy transport (migration) ability. Existing host materials include carbazole derivative,8–14 triphenylamine derivatives,15 triazine derivatives,16,17 and polyfluorenes.18,19 However, xanthone-based polymers have not been explored as a triplet host while xanthone has a T1 energy (3.21 eV)20,21 which is higher than that of blue-phosphorescent iridium bis[(4,6-difluorophenyl)pyridinato-N,C2]picolinate (FIrpic: T1 2.65 eV).9 Herein, we report the synthesis and properties of poly(xanthon-3-yl methacrylate) (poly(XOMA)) and poly(xanthon-3-yl methacrylate-co-methyl methacrylate) (poly(XOMA-co-MMA)) as well as xanthon-3-yl acetate (XA) as a model compound of monomeric unit (Chart 1). Polymers having xanthone moiety in the side chain have never been reported so far to the best of our knowledge. Even polymers having xanthone moiety in the main chain are limited to rather early examples such as a polymer made by Friedel–Crafts polymerization22a,b and a polyamide.22c
Results and discussion
The monomer, XOMA, was synthesized via condensation of 3-hydroxyxanthone with methacryloyl chloride. Radical polymerization of XOMA was carried out using α,α′-azobisisobutyronitrile (AIBN) in CHCl3 at different [M]0/[I]0 ratios in the dark in order to avoid xanthone-sensitized radical reactions (Table 1). The polymerizations resulted in almost quantitative conversions, and molar mass of the product can be moderately changed by altering [M]0/[I]0 ratio. The obtained polymers were soluble in solvents including CHCl3 and tetrahydrofuran. Poly(XOMA) (run 3 in Table 1) did not show a clear Tg and started to decompose at Tdecomp of 310.3 °C in thermal analyses.| Run | [M]0/[I]0 | Conv.b (%) | MeOH-insoluble polymer | ||
|---|---|---|---|---|---|
| Yield (%) | Mnc | Mw/Mnc | |||
| a XOMA = 100 mg (0.36 mmol) (runs 1, 2, and 4), 500 mg (1.8 mmol) (run 3).b Determined by 1H NMR (CDCl3).c Estimated by SEC on the basis of polystyrene standards (eluent: CHCl3).d Triad tacticity was mm/mr/rr = 8/40/52 as determined by 1H NMR of PMMA converted from the MeOH-insoluble part of poly(XOMA). | |||||
| 1 | 10 | >99 | 97 | 8500 | 5.52 |
| 2 | 25 | >99 | 92 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
6.03 |
| 3d | 50 | >99 | 92 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
8.32 |
| 4 | 100 | >99 | 92 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
5.03 |
Radical copolymerization of XOMA with methyl methacrylate (MMA) was also carried out at a feed monomer ratio of 1/1 at [MMA]0 = [XOMA]0 = 0.75 M, [AIBN]0 = 0.030 M, at 60 °C for 24 h to afford a copolymer of Mn 8440 and Mw/Mn 7.86 (SEC vs. standard polystyrene) and a unit ratio, [MMA]/[XOMA] in polymer of 0.50/0.50 was obtained. In SEC analyses using RI and UV (wavelength: 254 nm) detectors, the chromatograms obtained by the two detectors had very similar shapes (Fig. S4 in ESI†). These results indicate that two monomeric units are randomly distributed regardless of molar mass. The polymers is most probably a random copolymer.
Fig. 1 shows the 1H NMR spectrum of poly(XOMA) (run 3 in Table 1) along with those of XA as a model compound of monomeric unit and poly(MMA) derived from poly(XOMA). The spectrum of the poly(XOMA) indicated signals that are consistent with the polymer structure which was also supported by FT-IR and 13C NMR spectra (Fig. S2 in ESI†).
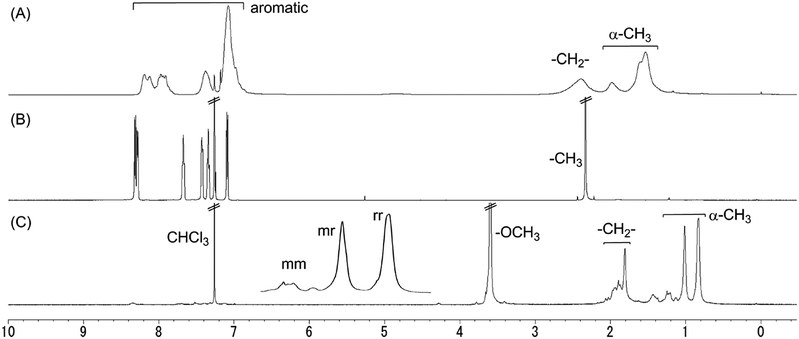 | ||
| Fig. 1 1H NMR spectra of poly(XOMA) (run 3 in Table 1) (A), XA (a unit model for poly(XOMA)) (B), and poly(MMA) converted from poly(XOMA) (run 3 in Table 1) (C). [400 MHz, CDCl3, 23 °C]. | ||
Tacticity of poly(XOMA) was assessed by 1H NMR spectra of poly(MMA) derived from the original polymer (Fig. 1C). Poly(XOMA) was converted to poly(MMA) by hydrolysis in MeOH containing NaOH followed by methylation using CH2N2. The 1H NMR spectrum of poly(MMA) indicated three separate signals of α-methyl group based on mm, mr, and rr triads. Poly(XOMA) obtained in this work is rich in syndiotacticity similarly to other polymethacrylates synthesized by free radical polymeriation.23
In the 1H NMR spectrum shown in Fig. 1A, the aromatic proton signals are slightly up-field shifted with respect to the corresponding signals of XA (Fig. 1B), which suggests π-stacking of the side-chain xanthone groups. Up-field shifts have been reported for poly(dibenzofulvene) and its derivatives having a regulated π-stacked conformation.24–26 Further, no major differences were confirmed between 1H NMR spectra taken at room temperature and at 55 °C in CDCl3; the proposed π-stacking seems to be maintained even at an elevated temperature (Fig. S5 in ESI†).
Fig. 2 shows UV-vis spectra of poly(XOMA) (run 3 in Table 1), poly(XOMA-co-MMA), and XA as a unit model. The poly(XOMA) indicated clear hypochromic effects with respect to XA, supporting that the side-chain groups are π-stacked. A connection between hypochromism and a π-stacked conformation has been established for DNAs27 and poly(dibenzofulvene) and its derivatives.24–26 If a polymer has a completely random conformation, hypochromic effects are not observed, which was confirmed in this work using polystyrene and ethylbenzene as a unit model (Fig. S6 in ESI†). Also, the stacking was found stable again at an elevated temperature through UV spectral measurements at various temperatures (Fig. S7 in ESI†). Further, poly(XOMA-co-MMA) showed weaker hypochromism compared with the poly(XOMA); the copolymer may have a less ordered π-stacked conformation. As the chromophores in the copolymer are rather randomly distributed, the chance of aligning two xanthone groups adjacent to each other should be less than that in the homopolymer.
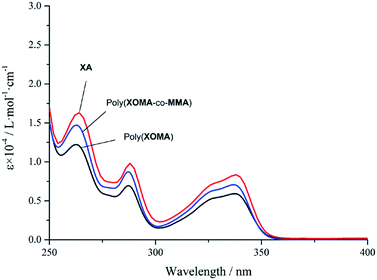 | ||
| Fig. 2 UV-vis spectra of XOMA polymers and a unit model in CHCl3: poly(XOMA) (run 3 in Table 1) (a), poly(XOMA-co-MMA) (b), and XA (c). [Conc. = 1.0 × 10−5 M, 23 °C]. | ||
The extent of overlapping of π-electronic systems of poly(XOMA) and poly(XOMA-co-MMA) showing no red shifts in the UV spectra may be smaller compared with that of poly(dibenzofulvene) showing remarkable red shifts.24–26 Otherwise, spatial arrangements of stacked chromophores may be different in poly(XOMA) or poly(XOMA-co-MMA) and in poly(dibenzofulvene). π-Stacked chromophores in DNA double helices shows clear hypochromicity and no clear red shifts.28
T1 energy transfer from poly(XOMA), poly(XOMA-co-MMA), and XA to FIrpic was examined in a CHCl3 solution at different concentrations where the concentration of polymer or XA was constant ([polymer or XA] = 1.0 × 10−5 M) and the concentration of FIrpic varied in the range of [FIrpic] = 0.11 × 10−5 to 1.0 × 10−5 M. Fig. 3A shows the absorbance and emission spectra of the solutions containing poly(XOMA) (run 3 in Table 1) and FIrpic. The spectra for the systems with poly(XOMA-co-MMA) and XA are found in ESI (Fig. S8 in ESI†). The emission spectra were recorded on excitation at 338 nm. The absorption spectral shape varied depending on the ratio of the polymer to FIrpic but emission spectral shape was unchanged and arose from FIrpic. The polymer alone hardly emitted at room temperature, which is consistent with the fact that xanthone has a high probability of intersystem crossing leading to a triplet state in excited states and emits clear phosphorescence only at low temperature.21,29
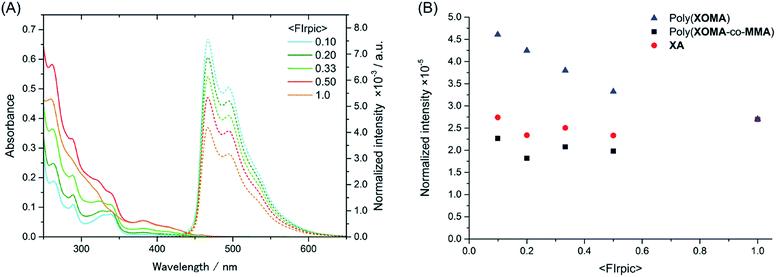 | ||
| Fig. 3 Absorbance and emission spectra of CHCl3 solution containing poly(XOMA) (run 3 in Table 1) and FIrpic (A), and a plot of normalized emission intensity against mole fraction of FIrpic for poly(XOMA) system, poly(XOMA-co-MMA) system, and control system with XA (B). The absorbance and emission spectra were taken in a 1 cm quartz cell at 23 °C at a constant [polymer (per unit residue)] of 1.0 × 10−5 M and various [FIrpic] in the range of 0.11 × 10−5 to 1.0 × 10−5 M for the data at <FIrpic> = 0.1–0.5. The spectrum at <FIrpic> = 1 corresponds to a pure FIrpic solution at [FIrpic] = 1.0 × 10−5 M. The absorbance and emission spectra of the poly(XOMA-co-MMA) system and the control system are in ESI (Fig. S8 in ESI†). | ||
Fig. 3B indicates a plot of normalized emission intensity against the mole fraction of FIrpic which is defined as <FIrpic> = [FIrpic]/([FIrpic] + [polymer (unit residue) or XA]). Since both xanthone moiety and FIrpic absorb at 338 nm and emission arises only from FIrpic, the emission spectral intensity was normalized to the contribution of FIrpic to absorbance at 338 nm which was calculated by multiplying absorbance by [FIrpic] × εFIrpic(338 nm)/([FIrpic] × εFIrpic(338 nm) + [polymer (unit residue) or XA] × (εpolymer(unit residue) or εXA(338 nm))) where εFIrpic(338 nm), εpolymer(338 nm), and εXA(338 nm) are molar absorptivities of FIrpic (8380 L mol−1 cm−1), polymers (poly(XOMA) 5900 L mol−1 cm−1; poly(XOMA-co-MMA) 7020 L mol−1 cm−1 (based on XOMA unit residue)), and XA (7750 L mol−1 cm−1), respectively, at 338 nm in CHCl3.
For the system with XA, normalized emission intensity of FIrpic is almost unchanged regardless of <FIrpic>. In contrast, for the system with poly(XOMA), normalized emission intensity of FIrpic was slightly greater than that of the pure FIrpic solution at <FIrpic> = 1. These results indicate that poly(XOMA) is excited and transfers triplet excited energy FIrpic (triplet energy harvesting) to but the degree of harvesting is rather small. The harvesting effect seems greater at a lower <FIrpic> which corresponds to a higher concentration of poly(XOMA). At a lower <FIrpic>, i.e., at a higher concentration of the polymer where interactions among polymer chains and those between a chain and FIrpic should be more frequent, T1 energy transferred through the Dexter mechanism30 requiring collision of between donor and acceptor species would be facilitated.
Although T1 energy of XA was estimated to be 3.43 eV by DFT calculations at the B3LYP method using 6-31G(d) basis set and is higher enough to be a sensitizer, it did not show harvesting effects. Further, poly(XOMA-co-MMA) did not harvest triplet energy either. These results suggest that π-stacked polymer structure24,31 where side-chain xanthone moieties are closely interacting with each other is important in attaining triplet energy harvesting.
The shape of the excitation spectrum of the poly(XOMA) solution at <FIrpic> = 0.10 was more similar to the absorption spectrum of a pure FIrpic solution at [FIrpic] = 1.0 × 10−5 M rather than to that of a pure polymer solution at [poly(XOMA) (per unit residue)] = 1.0 × 10−5 M (Fig. S9 in ESI†). This observation suggests that the observed emission from FIrpic arises mainly from directly excited FIrpic, which is consistent with the conclusion that the extent of the proposed harvesting effect by poly(XOMA) is rather small in solution.
The luminescence quantum yields of the pure FIrpic solution at <FIrpic> = 1 ([FIrpic] = 1.0 × 10−5 M) in CHCl3 was estimated to be 45% on excitation at 338 nm on the basis of a reported quantum yield in CH2Cl2 (62%, excitation at 407 nm)32 considering the differences in excitation wavelength and reflective index of the two solvents.33 In the same manner, the quantum yield of the solution containing poly(XOMA) and FIrpic at <FIrpic> = 0.1 ([FIrpic] = 1.0 × 10−6 M) was estimated to be 8% after normalization to the concentration of FIrpic at <FIrpic> = 1 ([FIrpic] = 1.00 × 10−5 M). Based on these quantum yields, the energy transfer efficiency was determined to be 11% at <FIrpic> = 0.1 (see ESI† for the definition of the energy transfer efficiency.). This means that 11% of photon energy absorbed by poly(XOMA) was transferred to FIrpic.
T1 energy transfer from poly(XOMA) and poly(XOMA-co-MMA) to FIrpic was further investigated in the solid state using film samples. Control experiments were performed using poly(MMA) which would not harvest triplet energy. Polymer films containing FIrpic at different mole fractions were fabricated on a quartz plate by a drop casting method, and their absorbance spectra and emission spectra on excitation at 338 nm were measured. Emission intensity was normalized to absorbance of FIrpic at 338 nm where light is absorbed both by polymer and FIrpic. FIrpic's contribution to absorbance at 338 nm was estimated by multiplying observed absorbance by <FIrpic> × εFIrpic(338 nm)/(<FIrpic> × εFIrpic(338 nm) + <polymer (unit residue)> × εpolymer(unit residue)) where <FIrpic> and <polymer (unit residue)> are mole fractions of FIrpic and polymer in a film, respectively, and ε values are those in CHCl3 solution.
The absorbance and emission spectra for the poly(XOMA) films are shown in Fig. 4A. The spectra for the other films are found in ESI (Fig. S10 in ESI†). The spectral patterns of the films were similar to those in solution, suggesting that the films are rather homogeneous and that photo physical properties are not significantly affected by diffraction and reflection effects. This is supported by the fact that the films were smooth and transparent (Fig. S11 in ESI†). Further, thicknesses of the films were ca. 0.12–0.19 μm where diffraction and reflection should not occur on irradiation at 338 nm and emission at around 450–600 nm (Table S2 in ESI†).
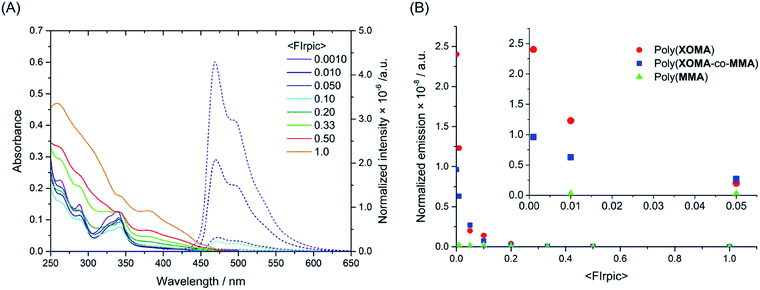 | ||
| Fig. 4 Absorbance and emission spectra of cast films made from poly(XOMA) (run 3 in Table 1) and FIrpic (A), and a plot of normalized emission intensity against mole fraction of FIrpic for the poly(XOMA) film, poly(XOMA-co-MMA) film containing FIrpic and control film made of poly(MMA) containing FIrpic (B). The absorbance and emission spectra of the poly(XOMA-co-MMA) films and the poly(MMA) films and the photographs and thickness data of the film samples are in ESI (Fig. S10 and S11 and Table S2 in ESI†). | ||
Normalized intensities of the film samples were plotted against <FIrpic> (Fig. 4B). The data point at <FIrpic> = 1 corresponds to a film sample fabricated using pure FIrpic. In the low <FIrpic> range (<0.01), films of poly(XOMA) emit light whose intensity is as much as 1000 times greater than that emitted by a pure FIrpic film sample on direct excitation or from the poly(MMA) films containing FIrpic, strongly indicating that poly(XOMA) very efficiently harvests triplet energy. The fact that emission intensity is greater at a lower <FIrpic> implies that direct interaction between FIrpic species hampers effective emission and that complete dispersion of FIrpic in a film is important in devising an efficient phosphorescent emitting material. In addition, poly(XOMA-co-MMA) also showed harvesting effects which were weaker than those of poly(XOMA), indicating that an ordered π-stacked structure of the poly(XOMA) plays an important role in energy harvesting in the solid state as well as in solution. Triplet energy migration between xanthone moieties may be remarkably enhanced by the stacked alignment of π-electron systems.
The shape of the excitation spectrum of the poly(XOMA) film at <FIrpic> = 0.010 was more similar to the absorption spectrum of the pure polymer film which has a clear absorption minimum at around 300 nm which is absent in the spectrum of the pure FIrpic film (Fig. S12 in ESI†). A minimum at around 300 nm is clearly seen in the excitation spectrum. These observations are in contrast to the results of the corresponding experiments in solution described earlier and indicate that the extent of energy harvesting by poly(XOMA) is much greater in the solid state than in solution.
Energy transfer efficiency estimation was attempted for the film samples in the same manner as applied for the solution samples; however, accurate estimation was difficult due to the fact that emission intensity seems not to be completely proportional to the concentration of FIrpic, especially at a higher <FIrpic>.
Effects of π-stacking on T1 energies were investigated by DFT calculations at the B3LYP method using the 6-31G(d) basis set for a unimer, meso (m) and racemo (r) dimer, and mm, mr, and rr trimer models of poly(XOMA) (Fig. S13 in ESI†). The m dimer and mm and mr trimers having tight, face-to-face π-stacked conformations before geometry optimization had much lower T1 energies compared with the unimer with the largest difference being −1.92 eV while rr trimer did not indicate a significant change in T1 energy. Similar effects of stacking on lowest singlet excited (S1) energies were observed. These results indicate that close stacking between adjacent units decreases excited energy. On the other hand, after optimization, the stacking of the poly(XOMA) models were much less tight, and effects of stacking on T1 and S1 levels became negligible, suggesting that in the real solution and film systems, the T1 level of poly(XOMA) would be maintained high enough so energy harvesting to FIrpic is feasible.
T1 level of poly(XOMA) was estimated by emission spectrum of the pure poly(XOMA) film to be 2.88 eV (a peak at 431 nm) (Fig. S14 in ESI†). This value is lower than that of xanthone20,21 but is higher than that of FIrpic.9 The film indicated a weak, broad emission in the range of ca. 400–700 nm which is much broader than the emission of xanthone dispersed in PMMA (ca. 380–520 nm).21 These results suggest that the emission from the poly(XOMA) film is based on excimer in triplet excited states and that T1 level is reduced by excimer formation.
In conclusion, poly(XOMA) was synthesized as a polymer having a xanthone moiety in the side chain for the first time and its triplet energy harvesting ability was evaluated. Poly(XOMA-co-MMA) was also prepared and characterized. Poly(XOMA) appears to have a π-stacked conformation and poly(XOMA-co-MMA) a less ordered one. Poly(XOMA) exhibited triplet energy harvesting effects for blue phosphorescent FIrpic where the effects were far greater in the solid state than in a CHCl3 solution. Poly(XOMA-co-MMA) also indicated harvesting effects in the solid state but the extent was lower than those of poly(XOMA). π-Stacked conformation thus plays an important role in triplet energy harvesting: energy migration among xanthone moieties in a chain would be facilitated by π-stacking.
Acknowledgements
This research was supported by MEXT program of Integrated Research on Chemical Synthesis. We thank Sumitomo Chemical for providing us with some of the chemicals for monomer synthesis.Notes and references
- C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1981, 51, 913 CrossRef PubMed.
- A. C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz and A. B. Holmes, Chem. Rev., 2009, 109, 897 CrossRef CAS PubMed.
- J. Liu, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2009, 109, 5799 CrossRef CAS PubMed.
- Y. Shirota and H. Kageyama, Chem. Rev., 2007, 107, 953 CrossRef CAS PubMed.
- P. F. H. Schwab, J. R. Smith and J. Mich, Chem. Rev., 2005, 105, 1197 CrossRef CAS PubMed.
- K. Walzer, B. Maennig, M. Pfeiffer and K. Leo, Chem. Rev., 2007, 107, 1233 CrossRef CAS PubMed.
- (a) M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151 CrossRef CAS PubMed; (b) D. F. O'Brien, M. A. Baldo, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 74, 443 Search PubMed; (c) M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 4 CrossRef CAS PubMed; (d) M. Segal, M. A. Baldo, J. J. R. Holmes, S. R. Forrest and Z. G. Soos, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 075211 CrossRef; (e) M. A. Baldo, D. F. O'Brien, M. E. Thompson and S. R. Forrest, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 14422 CrossRef CAS.
- J. S. Swensen, E. Policarpov, A. V. Ruden, L. Wang, L. S. Sapochak and A. B. Padmaperuma, Adv. Funct. Mater., 2011, 21, 3258 CrossRef.
- R. J. Holmes, S. R. Forrest, Y.-K. Tung, R. C. Kwong, J. J. Brown, S. Garon and M. E. Thonpson, Appl. Phys. Lett., 2003, 82, 2422 CrossRef CAS PubMed.
- C. Adachi, R. C. Kwong, P. Djurovich, V. Adamovich, M. A. Baldo, M. E. Thonpson and S. R. Forrest, Appl. Phys. Lett., 2001, 79, 2082 CrossRef CAS PubMed.
- A. van Dijken, J. J. A. M. Bastiaansen, N. M. M. Kiggen, B. M. W. Langeveld, C. Rothe, A. Monkman, I. Bach, P. Stössel and K. Brunner, J. Am. Chem. Soc., 2004, 126, 7718 CrossRef CAS PubMed.
- J. H. Park, T.-W. Koh, Y. Do, M. H. Lee and S. Yoo, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2356 CrossRef.
- Y. Tao, Q. Wang, C. Yang, Q. Wang, Z. Zhang, T. Zou, J. Qin and D. Ma, Angew. Chem., Int. Ed., 2008, 47, 8104 CrossRef CAS PubMed.
- (a) Y. Kawamura, S. Yanagida and S. R. Forrest, J. Appl. Phys., 2002, 92, 87 CrossRef CAS PubMed; (b) G. Rippen, G. Kaufmann and W. Klöpffer, Chem. Phys., 1980, 52, 165 CrossRef CAS.
- Y. Tao, Q. Wang, Y. Shang, C. Yang, L. Ao, J. Qin, D. Ma and Z. Shuai, Chem. Commun., 2009, 77 RSC.
- M. M. Rothmann, S. Haneder, E. D. Como, C. Lennartz, C. Schildknecht and P. Strohriegl, Chem. Mater., 2010, 22, 2403 CrossRef CAS.
- H.-F. Chen, S.-J. Yang, Z.-H. Tsai, W.-Y. Hung, T.-C. Wang and K.-T. Wong, J. Mater. Chem., 2009, 19, 8112 RSC.
- X. Gong, J. C. Ostrowski, G. C. Bazan, D. Moses, A. J. Heeger and M. S. Liu, Adv. Mater., 2003, 15, 45 CrossRef CAS.
- Z. Wu, Y. Xiong, J. Zou, L. Wang, J. Liu, Q. Chen, W. Yang, J. Peng and Y. Cao, Adv. Mater., 2008, 20, 2359 CrossRef CAS.
- (a) P. J. S. Gomes, C. Serpa and L. G. Arnaut, J. Photochem. Photobiol., A, 2006, 184, 228 CrossRef CAS PubMed; (b) M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, Hand book of photochemistry, CRC Press, Taylor & Francis Group, 3rd edn, 2006 Search PubMed; (c) H. J. Pownall and J. R. Huber, J. Am. Chem. Soc., 1971, 93, 6429 CrossRef CAS.
- M. Christoff and T. D. Z. Atvars, Macromolecules, 1999, 32, 6093 CrossRef CAS.
- (a) J. L. Patel and H. S. Patel, J. Macromol. Sci., Chem., 1986, A23, 635 CrossRef CAS; (b) J. L. Patel and H. S. Patel, J. Macromol. Sci., Chem., 1986, A23, 285 CrossRef CAS; (c) K. K. Mozgova, V. V. Korshak and S. G. Levitskaya, Plast. Massy, 1968, 10, 14 Search PubMed.
- (a) T. Nakano and Y. Okamoto, Controlled Radical Polymerization (ACS Symposium Series 685), American Chemical Society, Washington D.C., 1998, p. 451 Search PubMed; (b) T. Nakano and Y. Okamoto, Stereocontrolled Chiral Polymers, in Polymer Science: A Comprehensive Reference, ed. K. Matyjaszewski and M. Möller, Elsevier BV, Amsterdam, 2012, vol 6, p. 629 Search PubMed; (c) T. Nakano, A. Matsuda and Y. Okamoto, Polym. J., 1996, 28, 556 CrossRef CAS.
- (a) T. Nakano, K. Takewaki, T. Yade and Y. Okamoto, J. Am. Chem. Soc., 2001, 123, 9182 CrossRef CAS; (b) T. Nakano and T. Yade, J. Am. Chem. Soc., 2003, 125, 15474 CrossRef CAS PubMed.
- T. Nakano, T. Yade, M. Yokoyama and N. Nagayama, Chem. Lett., 2004, 33, 296 CrossRef CAS.
- T. Nakano, Polym. J., 2010, 42, 103 CrossRef CAS.
- (a) I. Tinoco, J. Am. Chem. Soc., 1960, 82, 4785 CrossRef CAS; (b) W. Rohdes, J. Am. Chem. Soc., 1961, 83, 3609 CrossRef.
- D. Voet, W. B. Gratzer, R. A. Cox and P. Doty, Biopolymers, 1963, 1, 193 CrossRef CAS.
- (a) R. Rusakowicz, G. W. Byers and P. A. Leermakers, J. Am. Chem. Soc., 1971, 93, 3263 CrossRef; (b) J. C. Scaiano, J. Am. Chem. Soc., 1980, 102, 7747 CrossRef CAS; (c) M. Barra, C. Bohne and J. C. Scaiano, J. Am. Chem. Soc., 1990, 112, 8075 CrossRef CAS.
- D. L. Dexter, A theory of sensitized luminescence in solids, J. Chem. Phys., 1953, 21, 836 CrossRef CAS PubMed.
- (a) Y. Morisaki and Y. Chujo, Angew. Chem., Int. Ed., 2006, 45, 6430–6437 CrossRef CAS PubMed; (b) Y. Morisaki and Y. Chujo, Prog. Polym. Sci., 2008, 33, 346–364 CrossRef CAS PubMed.
- E. Baranoff, B. F. E. Curchod, F. Monti, F. Sterimer, G. Accorsi, I. Tavernelli, U. Rothlisberger, R. Scopelliti, M. Grätzel and M. K. Nazeeruddin, Inorg. Chem., 2012, 51, 799 CrossRef CAS PubMed.
- (a) H. Ishida, S. Tobita, Y. Hasegawa, R. Katoh and K. Nozaki, Coord. Chem. Rev., 2010, 254, 2449 CrossRef CAS PubMed; (b) D. F. Eaton, Pure & Appl. Chem., 1988, 60, 1107 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, spectrometric data of compounds, and film properties. See DOI: 10.1039/c4ra16023a |
| This journal is © The Royal Society of Chemistry 2015 |