Two-phase mixture model for substrate degradation and photo-hydrogen production in an entrapped-cell photobioreactor under various light intensities
Abstract
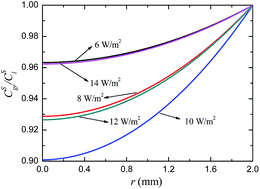
A two-phase mixture model was developed for revealing the interaction between substrate degradation and photo-hydrogen production in an entrapped-cell photobioreactor under various light intensities. The effects of the porosity of the packed bed and the height of the photobioreactor on the substrate degradation rate (SDR) and hydrogen production rate (HPR) were also predicted at different light intensities.

 Please wait while we load your content...
Please wait while we load your content...