Structural properties of cerium doped siloxane–PMMA hybrid coatings with high anticorrosive performance
Abstract
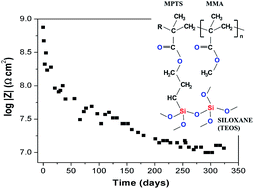
This study reports on the characterization of cerium doped organic–inorganic hybrid coatings by correlating their structural properties with the corrosion protection efficiency of coated carbon steel. The films were prepared via the sol–gel route from radical polymerization of methyl methacrylate (MMA) with 3-methacryloxy propyltrimethoxysilane (MPTS) followed by acidic hydrolysis and condensation of tetraethoxysilane (TEOS). After identifying the optimum proportion of polymeric and silica phase for the formation of a highly ramified structure (MMA/TEOS = 4), increasing concentration of Ce(IV) ions (0.1% < Ce/Si < 5%) was added to the inorganic precursor to enhance the passivating character of the films. Nuclear magnetic resonance, X-ray photoelectron spectroscopy, small angle X-ray scattering, atomic force microcopy and thermogravimetry were used to characterize the structural properties and electrochemical impedance spectroscopy, potentiodynamic polarization curves and field emission scanning electron microscopy were employed to evaluate the corrosion resistance of the coated samples in a standard saline environment. Structural analysis revealed a high degree of connectivity of the hybrid network, resulting in an enhanced thermal and electrochemical stability of the material. Detailed investigation of the structural effect of cerium species revealed that reduction of Ce(IV) ions during the polycondensation process catalyzes an increase of siloxane network connectivity (>87%) and enhances the polymerization of organic moieties. The electrochemical assays showed that the coatings possess an excellent long-term stability at high corrosion resistance of up to 10 GΩ cm2 and current densities about 6 orders of magnitude lower than that of the bare carbon steel. For elevated Ce(IV) doping levels the self-healing ability was observed preventing during long-term exposure further progression of the corrosion process.

 Please wait while we load your content...
Please wait while we load your content...