Titania synthesized through regulated mineralization of cellulose and its photocatalytic activity
Irina Postnovaab,
Ekaterina Kozlovacd,
Svetlana Cherepanovacd,
Sergei Tsybulyacd,
Andrey Rempele and
Yury Shchipunov*a
aInstitute of Chemistry, Far East Department, Russian Academy of Sciences, 690022 Vladivostok, Russia. E-mail: YAS@ich.dvo.ru; Fax: +7 423 2348353; Tel: +7 423 2314481
bChemical Department, Far-East Federal University, Vladivostok 690950, Russia
cInstitute of Catalysis, Siberian Department, Russian Academy of Sciences, Novosibirsk, 630090, Russia
dResearch and Educational Center for Energy Efficient Catalysis, Novosibirsk State University, Pirogova 2, Novosibirsk, 630090, Russia
eInstitute of Solid State Chemistry, Ural Department, Russian Academy of Sciences, Ekaterinburg, 620990, Russia
First published on 24th December 2014
Abstract
Cellulose used for thousands of years has been rediscovered recently as a novel smart material for various nanotechnological applications. Its insoluble fibrils are functionalized by using mineralization methods developed in nanochemistry. Here they are coated by titania synthesized in one stage by a new green approach. It consists of controlling the localization of very fast hydrolysis and condensation reactions. Cellulose fibrils are placed in ethylene glycol with such an amount of water that is absorbed entirely by the hygroscopic polysaccharide. This hydrating water works as a reaction centre when the precursor reaches it. Instant hydrolysis and following condensation reactions proceeding mainly on the fibrils provide their mineralization. Titania prepared at ambient conditions is in an amorphous state. It is transferred in crystalline forms under a variety of conditions including moderate temperature (80 °C), calcination in air and cellulose carbonization in an inert atmosphere. These treatments result in photocatalytic activity. Even cellulose treated at the moderate conditions demonstrates significant self-cleaning ability consisting of fast degradation of methylene blue under outdoor sunlight irradiation. Photocatalytic activity of carbon–titania hybrids includes a side reaction of the oxidation of the carbonized fibrils. Photocatalytic properties of some of the calcinated samples, not containing organics, were comparable with a commercial photocatalyst.
Introduction
Titanium dioxide TiO2 or titanium(IV)oxide and simple titania cause tremendous interest because of their great potential for developing very efficient photocatalysts, solar-energy converters, ductile ceramics, sensors and self-cleaning textiles.1–7 They also possess high chemical stability and low toxicity. Nowadays, TiO2 is one the most widely used photocatalysts. Titania provides controlled photocatalytic reactions for organic synthesis, photocatalytic oxidation of organic compounds, and photocatalytic hydrogen production. Its efficacy is highly dependable on the crystallinity and crystalline form of TiO2, its shape and nanodimension. Various routes are suggested to prepare nanosized titania of different shape and dimensionality by non-hydrolytic hydrothermal and hydrolytic sol–gel processing by using surfactant-mediated and template syntheses.2,6,8–10 There are numerous attempts to use biopolymers for the templating metal oxides.11–15 One of them attracting much current attention for the mineralization is cellulose. Its coating by titania offers the prospects for the modification of surface properties and imparting new functions to the fabrics interesting for the textile industry.7,16–18 Titania on cellulose demonstrates various activities like stain removal and self-cleaning surface via the photocatalytic degradation, antibacterial deodorizing, UV blocking, flame retardant, and deNOx functions. It can also provide both surface hydrophobization and hydrophilization.2,4Current dominant coating technique for cellulose are so-called dip-pad–dry-cure and dip-coating methods.18–23 It is performed with separately prepared TiO2 sol nanoparticles. Fabric is padded simply with a sol solution. Typical disadvantage of this technique is in the inadequate adhesion of titania with biomacromolecules.7,24 Attempts to prepare TiO2 directly on cellulose were done in ref. 25 and 26. The precursor was dripped in cellulose-containing aqueous solution under the ultrasonic irradiation. Attainment of this aim is in doubt because of the fast hydrolysis–condensation reactions starting instantly after the contact of precursor with water. Evidence for that followed from loose coating on the fibers.26
Kunitake with Huang in ref. 27 provided first the absorption of precursor on cellulose fibers by passing its nonaqueous solution. When water flowed subsequently through this treated sample, it promoted the instant hydrolysis and condensation reactions resulting in the TiO2 formation. This method reproduced then in ref. 28 was used only for the mineralization of filter paper.
The preparation of titania on the cellulose fibers by the most above-considered routes did not provide sufficient binding of TiO2 with polysaccharide. It causes the poor operational stability and durability of bionanocomposite materials. To improve the stability of titania coating, an additional cross-linking procedure was suggested to use in ref. 24. Poly-carboxylic acids attached covalently to cellulose fiber surface allowed binding titania through electrostatic interactions with free carboxylic groups. These cross-links improved notably functional properties of textile with TiO2 coating.29 Carboxylic groups were also introduced directly on cellulose surface by using radio frequency plasma, microwave plasma and vacuum-UV irradiation treatment.21 Under these conditions the chemical degradation of fibers took place as well that can decrease the durability of textile.
The problem of insufficient binding of TiO2 with carbohydrate macromolecules was got around in our recent article.30 We suggested a one-stage simple procedure in which the formation of TiO2 proceeded directly on biomacromolecules. Its certain localization was provided by means of the addition of water in such amount that was needed only to hydrate functional groups of hygroscopic polysaccharides, while the H2O was absent in the bulk of solution prepared on the basis of an organic solvent (ethylene glycol). Because the instant hydrolysis and condensation reactions took place only after the contact of precursor with water molecules, the formation of TiO2 was localized on the hydrated functional groups. Furthermore, titania generated in situ could enter into reactions with hydroxyl groups on polysaccharide macromolecules, binding covalently with them. Experiments with the mineralization of xanthan and sodium hyaluronate soluble in ethylene glycol showed that this enabled the TiO2 structure to be manipulated by varying the concentrations of water, precursor and polysaccharides in the reaction system.30,31 Among the advantages of the approach was the formation of monolithic titania.
Here our approach is extended for the mineralization of cellulose fibrils consisting of tightly associated carbohydrate macromolecules. It was demonstrated in ref. 31 that they could serve as the template for titania instead of soluble polysaccharides. Cellulose possessed considerable merit because of its various forms like fibrils, mats and pads practically usable after their mineralization. They are rather mechanically strong and flexible. In addition, cellulose can be carbonized or completely removed by means of calcination. It is our aim to demonstrate these advantages via the direct formation of titania on cotton pads by the new approach, its transformation under various thermal treatments and corresponding photocatalytic activity including self-cleaning ability.
Results and discussion
A Synthesis of titania
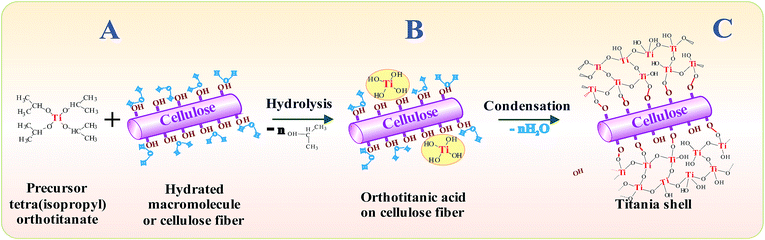
One-pot preparation of titania includes three main steps. The procedure is illustrated with explanatory drawings in Fig. 1.| Ti(–OR)4 + nH2O → (HO–)nTi(–OR)4−n + nHO–R | (1) |
| (RO–)4−nTi(–OH)n + (HO–)nTi(–OR)4−n → (OH)n−1(RO–)4−nTi–O–Ti(–OR)4−n(OH)n−1 + H2O | (2) |
| Ti(–OR)4 + (HO–)nTi(–OR)4−n → (RO–)3Ti–O–Ti(–OR)4−n(OH)n−1 + HO–R | (3) |
Circumstantial evidence of the localization of processes leading to the titania formation on cellulose fibrils followed from the observation of the retention of optical transparency at the early stage of the precursor addition. When taking only ethylene glycol with even tiny amount of added H2O, one could find a near-instant appearance of precipitate in its bulk after the introduction of TIPOT. In the presence of hydrated cellulose the opalescence developed with time. The greater the hydration, the earlier the appearance of opalescence. A solution could remain optically transparent over few hours when it was not shaken or stirred. This observation points to the absence of free water in the bulk, being mainly in hydrated shells of polysaccharide functional groups.
It should be mentioned that orthotitanic acid generated after the precursor hydrolysis in accordance with eqn (1) can enter into reactions like the condensation one (2) also with hydroxyl groups at the surface of cellulose fibrils:32
 | (4) |
The covalent bonds thus formed will provide the rigid attachment of titania to polysaccharide (Fig. 1C). This is a fundamental advantage of our approach over the dip-coating and dip-pad–dry-cure techniques in which independently synthesized titania nanoparticles are mechanically deposited on cotton by padding.19–23
B Titania morphology
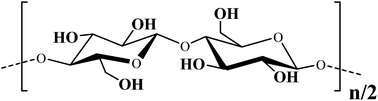
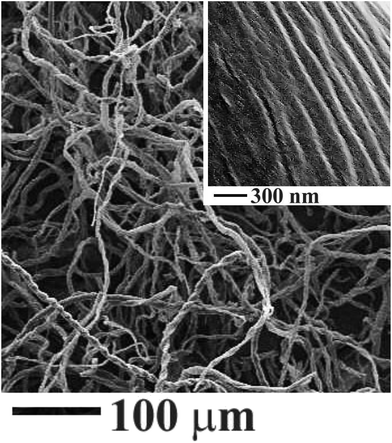
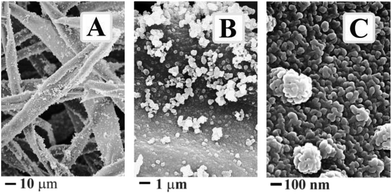
Cellulose, of which structural formulae is shown in Fig. 2, is not soluble in ethylene glycol. Its linear macromolecules are firmly associated through numerous hydrogen bonds that keep solvent from penetrating between them.33 Water cannot penetrate as well. One may observe the swelling of cotton pads without breaking apart. They were in pristine state after the hydration, i.e., in the form of a sheet from entangled microscopic fibrils (Fig. 3) with hydrated water sitting mainly on their surface. Fibrils are visible to the naked eye but they compose of smaller ones (inset, Fig. 3).33 They have an expanded surface area and highly porous matrix convenient for development of composite materials.34,35Fig. 4 presents a set of representative FESEM images of cellulose fibers treated by the foregoing method. When comparing them with the pictures in Fig. 3, one may recognize the presence of a titania coating. It seems not dense from Fig. 4A and B. These images characterize the surface morphology at the microscopic level. As obvious from the picture taken at larger magnification with better resolution (Fig. 4C), there are dense coating consisting of rather uniform nanoparticles. They form the initial shell. There are discrete micro/nanoparticles at its surface (Fig. 4A and B). They were generated on the initial coating, presenting the secondary nonuniform arrays. The SEM images in Fig. 4 unambiguously point to the titania formation on the cellulose fibrils as the coating from nanoparticles packed closely together.
Formation of titania coating occurred instantly after the contact of precursor with water being in the hydration shells of hydroxyl groups (Fig. 1B and C). Of fundamental importance of the approach used is the H2O amount introduced. It should be added in a limited extent sufficient only for the cellulose hydration. This important feature was revealed first when soluble polysaccharides were taken. The water absence in the bulk of solution provides its certain location and, as a consequence, the localization of reactions (1)–(3). The titania formation proceeded mainly on the hydrated groups serving as centers for the cellulose mineralization. Because the fibril surface is covered by numerous hydroxyls, this is the chief cause for the formation of uniform dense coating (Fig. 4C).
C Crystalline titania
The synthesis by the developed approach was performed at the ambient conditions. Titania in this instance was obtained in amorphous state. This is a common case. To transfer it in the crystalline state, TiO2 is frequently put through a thermal post-treatment.2,4,8,9 Cellulose is a natural polymer with the restricted thermal stability.33 It was treated at 80 °C for 12 h as a precautionary measure against polysaccharide degradation (see the TGA data and corresponding discussion below). Examination of a sample thus treated under the TEM revealed the presence of nanoclusters of crystalline titania in the coating. One may see them in the TEM picture presented in Fig. 5. There are a few separated nanocrystals not exceeding 5 nm in dimension.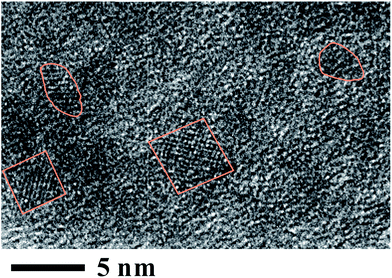 | ||
| Fig. 5 TEM image of crystalline titania synthesized on cellulose fibrils and then treated at 80 °C in air. Nanocrystallites are surrounded by lines. | ||
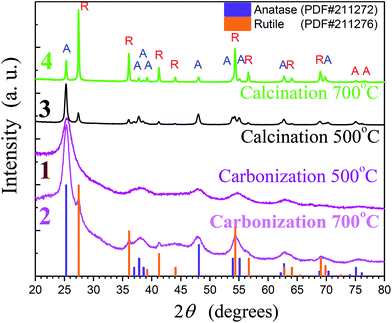
Crystallinity, yield and dimensions of crystalline titania are increased with increasing the temperature. For example, the sample, an image of which are shown in Fig. 6A, was calcinated in inert, argon atmosphere at 300 °C. One may see larger crystalline titania nanoparticles than that in the previous Fig. 5. There are distinct lattice planes with indexed spacings d = 0.355 nm and d = 0.325 nm. They bear on the 〈101〉 lattice plane of anatase and the 〈110〉 lattice planes of rutile, respectively. Both the crystalline forms were formed in the course of calcination but the anatase was in large excess compared to rutile. This sample consisting of carbon coated by TiO2 refers to composite or hybrid materials. The metal oxide did not crumble away when was calcinated although the transition of less-packed amorphous form into the close-packed crystalline structures went on in the course of treatment.36 This fact is evidence of its good adhesion to cellulose fibers owing to the covalent bonding to it (Fig. 1C).
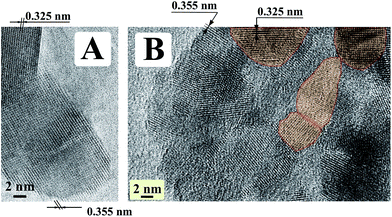 | ||
| Fig. 6 TEM images of crystalline titania synthesized on cellulose fibrils and then calcinded at 300 °C in argon atmosphere (A) and at 500 °C in air (B). | ||
An alternative way of the heating post-treatment is in air. Instead of the above-considered carbonization of organic template, it was removed by the oxidation. Titania free of carbon was prepared as well. It was also in crystalline state as follows from a representative TEM image in Fig. 6B. Polycrystalline structure can be identified from the random orientation of several lattice planes. Examination showed the presence of anatase according to the spacing d = 0.355 nm. It was indexed only in one case but this crystalline form was in the majority. According to our estimates from X-ray diffraction data presented below there were 84 wt% of anatase, the remainder (16 wt%) being rutile. Its 〈110〉 lattice planes with d = 0.325 nm are marked off by the red. The average nanocrystallite size determined from the broadening of the (110) and (101) reflections (see Fig. 7) by the Scherrer formula are approximately 32 and 35 nm, respectively, for anatase and rutile.
Heat treatment in the air (calcination) or inert atmosphere (carbonization) resulted in some differences in the crystallinity of titania. The calcinated TiO2 did not include organics or carbon, while the carbonized samples may be referred to hybrids (Fig. 5). A comparison of their X-ray patters in Fig. 7 revealed a notable difference in the anatase/rutile ratio in the coating prepared at the same temperature. One may see a larger content of rutile relative to anatase (curves 3 and 4) in the calcinated sample than in carbonized one (curves 1 and 2). The difference might be caused by an influence of the cellulose fibers on which surface the titania was formed (Fig. 1C and 4). Heat treatment in air resulted in the oxidative degradation of polysaccharide before the anatase → rutile transformation, whereas the substrate was not eliminated in the inert atmosphere. In this case, the covalent linkages of titania with cellulose fibrils (Fig. 1C) could hamper TiO2 conversion from one to another crystalline state that amount of anatase in samples calcinated at 500 and 700 °C decreased from 84 to 26 wt%, respectively. The remainder was rutile in both the cases. Anatase and rutile are the main crystalline forms of TiO2.8,36 The former is prepared at lower temperature than the latter. Therefore, we observed mainly the formation of anatase at ≤500 °C, then rutile with the temperature increasing up to 700–900 °C. A similar situation took place in studying with ethylene glycol-soluble polysaccharides.30,31
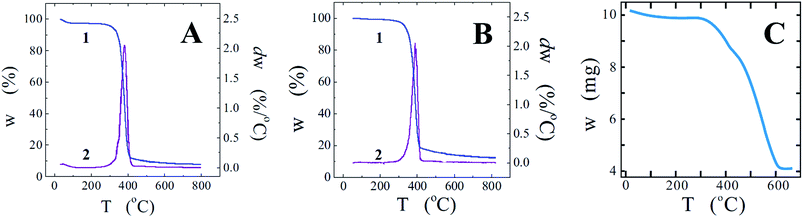
Heat treatment of titania is attended with cellulose degradation/carbonization. Crystalline TiO2 can be prepared at the temperature below 100 °C as well (Fig. 5) but prolonged heating time is needed.2 The crystallinity is improved and the treatment duration is decreased with increasing the temperature. Cellulose is considered as a polymer of moderate thermal stability.33 Its decomposition starts at around 200 °C. We applied thermogravimetric (TGA) analyses to examine the thermal stability of the studied cellulose in the inert atmosphere (nitrogen). There is a small weight loss observed in a dependence of weight vs. the temperature between 40–80 °C (curve 1, Fig. 8A). It can be assigned to the removal of water absorbed in cellulose. More pronounced changes take place in the range of 300–420 °C beginning at 255 °C with a peak in the derivative thermogravimetric curve 2 at 380 °C. The remains (ca. 7.8%) found after the cellulose decomposition is the carbon. The TGA characteristic presented in Fig. 8A bears close similarities to that in publications of another authors (see, e.g. ref. 37).
Fig. 8B presenting also TGA data serves to characterize if the mineralization of cellulose fibrils has or not an effect on their thermal degradation. As followed from the comparison with Fig. 8A, there are some differences between two plots but they are not so significant. The degradation started at 278 °C (curve 1), while a peak in the curve 2 is at 388 °C. The delayed beginning means that the titania coating occurred some shielding at the initial stages but its effect became less obvious with increasing the temperature.
To determine the amount of titania on carbonized fibrils, the TGA measurements were carried out in air. As seen from Fig. 8C, there were about 40% of TiO2 after the carbon removal. This is in good agreement with the amount found from the graphs in Fig. 8A and B. We had got as much as 7.8 and 12.4% of the remains after the carbonization of cellulose and its mineralized sample, respectively.
D Photocatalytic activity
In the beginning, mineralized cotton pads treated at 80 °C for 8 h were examined. This gentle temperature treatment did not result in any degradation of cellulose fibrils. It follows from the TGA results (Fig. 8). They were similar in appearance to the initial pads in spite of the titania coating. Its presence was confirmed by SEM and TEM microscopy (Fig. 5). When 0.01% solution of methylene blue dye was dropped onto the sample surface, it was sucked inside. The same amount was used in a case of non-mineralized, pristine cotton pads that served as controls. The presence of dye was obvious from the appearance of blue color (Fig. 9). We put the samples for exposing to the outdoor sunlight irradiation. It was found that the dye was discolored in the mineralized cellulose within 10 min although negligible discoloration was observed in the control sample. These results are indicative of high photocatalytic activity of titania coating on cellulose fibers and their efficient self-cleaning ability. Authors, who coated cellulose by TiO2 under the ultrasonic irradiation, observed the methylene blue discoloration within 20 h.26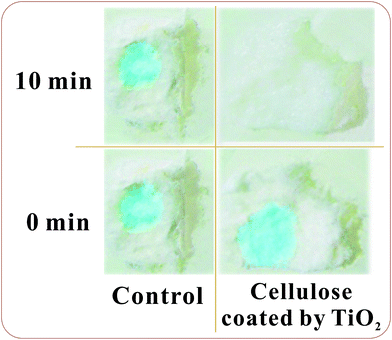 | ||
| Fig. 9 Pictures of samples of TiO2 coated cellulose and pristine cellulose with methylene blue before and after the 10 min outdoor sunlight irradiation. | ||
Calcinated and carbonized samples were examined as well. A fundamental difference between them was found. When carbonized titania was examined, the solution of methyl orange used to determine its photocatalytic activity did not become colourless although an active gas release was observed. This fact meant that there was the photocatalytic oxidation, but mainly of carbonized substrate as a side reaction. The possibility of oxidative decomposition of cellulose promoted by coated titania was also mentioned by other researchers (see, e.g., ref. 7, 32 and 35).
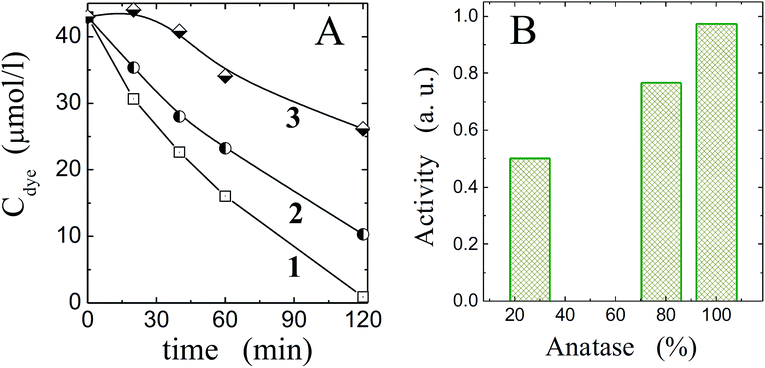
Titania free of polysaccharide template eliminating at 500 °C in air could demonstrate rather high photocatalytic activity against the methyl orange. Some results are shown in Fig. 10A (curves 2 and 3). When they are compared in activity with the commercial highly reactive photocatalyst like Hombifine N (curve 1, Fig. 10), the proximity of one sample (curve 2) to it is apparent. Another presenting by the curve 3 was worse. In some instances, the photocatalysis was at minimum level. It is the well-known fact of dependence of titania photocatalytic properties on the various factors.1,2,4,5 As was found, the synthesis conditions, temperature treatment, nanocrystalline dimension and crystalline form influenced notably on the photocatalysis. One may see in Fig. 10B an effect of anatase content on the photocatalytic activity. It was also regulated in pronounced manner by nanocrystalline dimension that in its turn were determined by the synthesis conditions such as the water and titania precursor contents, their molar ratio, duration of synthesis and post-synthetic treatment. To improve the photocatalytic properties of TiO2 synthesized by the suggested approach, it is necessary to establish the correlation between the mentioned factors.
Experimental
Materials
Tetra(isopropyl)orthotitanate (TIPOT) supplied by ABCR or gifted by Protek (Russia) was used as received. It was low viscous liquid being optically transparent that bears witness to their good isolation from the contact with atmosphere. Pads of 100% purified cotton without any fluorescent additives of the size of 50 × 60 mm (Thefaceshop, Korea) were used for cellulose. Ethylene glycol (reagent grade) was distilled prior to the stock solution preparation.The cotton pads thus mineralized were put through various heat treatment to provide the titania conversion in crystalline state. The minimal temperature was 80 °C at which it was left for 8 h. The samples were also calcinated in a muffle furnace at 500 °C over 5 h. A portion of them was treated at 300, 700 and 900 °C. On heating, two stops were made at 100 and 150 °C lasted for 30 and 60 min, respectively. The calcination was made both in air and inert atmosphere (argon).
Material characterization
Observations in the transmission mode were performed with a JEM-2010 high-resolution transmission electron microscope (TEM, JEOL, Japan) at an accelerating voltage of 200 kV. The samples were prepared by the dispersion of synthesized titania in ethanol. Few droplets of the solution were deposited on the copper grid with a carbon film. It was dried under the hot air blast.
The quantitative test was performed with methyl orange dye. Its 4.20 μmol was dissolved in 100 ml of water in which 120 mg grinded TiO2 was then dispersed. The mixture was irradiated with a high-pressure mercury vapor lamp DRSH-1000 (Russia) of 16 mW cm−2. Aliquots were taken from the reaction system at regular intervals to determine the concentration of methyl orange by spectrophotometrically at 465 nm by means of a UV/VIS Lambda 35 spectrophotometer (Perkin Elmer). For reference of the synthesized TiO2, the industrial photocatalyst Hombifine N (Sachtleben Chemie, GmbH) was taken.
Conclusions
Mineralization of cellulose fibrils by titania was performed in control mode by means of a novel approach. Titania synthesis is characterized by too fast hydrolysis and condensation reaction started immediately after the precursor contact with water. To control their localization directly on the cellulose macromolecules, they were hydrated in such manner that water was absent in solution bulk. As a media for the syntheses, ethylene glycol served which did not have significant effect on the titania formation. Owing to the almost instant hydrolysis and condensation reactions, TiO2 precipitated mainly on hydrated cellulose, providing its mineralization in one stage in situ at ambient conditions. Titania being in amorphous state after the synthesis was transferred into a crystalline form – anatase, rutile or their mixture by post-synthetic heating treatment. Photocatalytically active TiO2 prepared at the moderate temperature (80 °C) provided fast self-cleaning of cellulose under the outdoor sunlight irradiation. Calcination in air or carbonization in argon resulted in TiO2 free of organic or its hybrids with carbonized fibrils, respectively. The former demonstrated rather high photocatalytic activity of which level was comparable with commercial photocatalyst. The latter was also active under the UV irradiation but it was caused by oxidative degradation of carbon substrate on which it was prepared.Acknowledgements
This work was supported partially by the Russian Foundation for Basic Research (Project # 12-03-00726a), the Far East Branch of the Russian Academy of Sciences (Project # 15-I-3-006), the Siberian Branch of the Russian Academy of Sciences (Project 35) and the Ural Branch of the Russian Academy of Sciences (Project 12-S-3-1002).Notes and references
- A. Mills and S. Le Hunte, J. Photochem. Photobiol., A, 1997, 108, 1 CrossRef CAS.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891 CrossRef CAS PubMed.
- A. A. Rempel, Russ. Chem. Rev., 2007, 76, 474 CrossRef PubMed.
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515 CrossRef CAS PubMed.
- U. I. Gaya and A. H. Abdullah, J. Photochem. Photobiol., C, 2008, 9, 1 CrossRef CAS PubMed.
- M. Niederberger and N. Pinna, Metal oxide nanoparticles in organic solvents. Synthesis, formation, assembly and application, Springer, London, 2009 Search PubMed.
- M. Radetic, J. Photochem. Photobiol., C, 2013, 16, 62 CrossRef CAS PubMed.
- C. J. Brinker and G. W. Scherer, Sol–gel science. The physics and chemistry of sol–gel processing, Academic Press, Boston, 1990 Search PubMed.
- A. C. Pierre, Introduction to sol–gel processing, Kluwer, Boston, 1998 Search PubMed.
- Y. Shchipunov and A. Krekoten, Colloids Surf., B, 2011, 87, 203 CrossRef CAS PubMed.
- H. Golfen and S. Mann, Angew. Chem., Int. Ed., 2004, 42, 2350 Search PubMed.
- Handbook of biomineralization. Biomimetic and bioinspired chemistry, ed. P. Behrens and E. Bauerlein, Wiley-VCH, Weinheim, 2007 Search PubMed.
- Q. Dong, H. Su, W. Cao, D. Zhang, Q. Guo and Y. Lai, J. Solid State Chem., 2007, 180, 949 CrossRef CAS PubMed.
- M. B. Dickerson, K. H. Sandhage and R. R. Naik, Chem. Rev., 2008, 108, 4935 CrossRef CAS PubMed.
- Y. Shchipunov, Pure Appl. Chem., 2012, 84, 2579 CrossRef CAS.
- G. Goncalves, P. A. A. P. Marques, R. J. B. Pinto, T. Trindade and C. P. Neto, Compos. Sci. Technol., 2009, 69, 1051 CrossRef CAS PubMed.
- R. Dastjerdi and M. Montazer, Colloids Surf., B, 2010, 79, 5 CrossRef CAS PubMed.
- W. S. Tung and W. A. Daoud, J. Mater. Chem., 2011, 21, 7858 RSC.
- W. A. Daoud and J. H. Xin, J. Am. Chem. Soc., 2004, 87, 953 CAS.
- M. Uddin, F. Cesano, F. Bonino, S. Bordiga, G. Spoto, D. Scarano and A. Zecchina, J. Photochem. Photobiol., A, 2007, 189, 286 CrossRef CAS PubMed.
- T. Yuranova, D. Laub and J. Kiwi, Catal. Today, 2007, 122, 109 CrossRef CAS PubMed.
- K. Nelson and Y. Deng, Langmuir, 2008, 24, 975 CrossRef CAS PubMed.
- Y. Zhang, S. Li, F. Huang, F. Wang, W. Duan, J. Li, Y. Shen and A. Xie, Russ. J. Phys. Chem. A, 2012, 86, 413 CrossRef CAS.
- K. T. Meilert, D. Laub and J. Kiwi, J. Mol. Catal. A: Chem., 2005, 237, 101 CrossRef CAS PubMed.
- I. Perelshtein, G. Applerot, N. Perkas, J. Grinblat and A. Gedanken, Chem.–Eur. J., 2012, 18, 4575 CrossRef CAS PubMed.
- F. A. Sadr and M. Montazer, Ultrason. Sonochem., 2014, 21, 681 CrossRef PubMed.
- J. Huang and T. Kunitake, J. Am. Chem. Soc., 2003, 125, 11834 CrossRef CAS PubMed.
- Y. Luo, J. Xu and J. Huang, CrystEngComm, 2014, 16, 464 RSC.
- L. Karimi, M. Mirjalili, M. E. Yazdanshenas and A. Nazari, Photochem. Photobiol., 2010, 86, 1030 CrossRef CAS PubMed.
- Y. A. Shchipunov and I. V. Postnova, Colloids Surf., B, 2009, 74, 172 CrossRef CAS PubMed.
- I. V. Postnova, A. V. Krekoten, E. A. Kozlova, S. V. Tsybulya, A. A. Rempel and Y. A. Shchipunov, Russ. Chem. Bull., 2013, 62, 976 CrossRef CAS PubMed.
- J. Huang and Y. Gu, Curr. Opin. Colloid Interface Sci., 2011, 16, 470 CrossRef PubMed.
- D. Klemm, B. Philipp, T. Heinze, U. Heinze and W. Wagenknecht, Comprehensive cellulose chemistry. Fundamentals and analytical methods, Wiley-VCH, Weinheim, 1998 Search PubMed.
- G. Siqueira, J. Bras and A. Dufresne, Polymers, 2010, 2, 728 CrossRef CAS PubMed.
- Z. Schnepp, Angew. Chem., Int. Ed., 2013, 52, 1096 CrossRef CAS PubMed.
- R. J. D. Tilley, Crystals and crystal structure, Wiley, Chichester, 2006 Search PubMed.
- B. Wielage, T. Lampke, G. Marx, K. Nestler and D. Starke, Thermochim. Acta, 1999, 337, 169 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |