A novel Z-scheme WO3/CdWO4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of organic pollutants†
Abstract
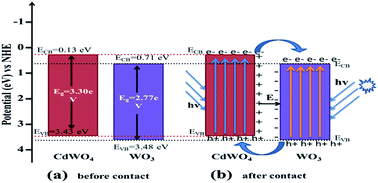
A novel Z-scheme WO3/CdWO4 photocatalyst has been fabricated with sheet-like tungsten trioxide (WO3) hybridized by rod-like cadmium tungstate (CdWO4) via a hydrothermal and chemisorption method. The as-synthesized WO3/CdWO4 photocatalyst exhibited enhanced photocatalytic efficiency for the degradation of different organic dyes under visible light irradiation. It was found that the photocatalytic performance of the composite WO3/CdWO4 was much higher than that of either WO3 or CdWO4 for the degradation of each organic dye. The highest activity of the composite was recorded for the degradation of MB which was about 7 times greater than pure CdWO4 and 2.3 times that of pure WO3. The enhanced performance of the photocatalyst was mainly attributed to the increased surface area and introduction of WO3 into the composite sample, which can induce higher adsorption activity for organic dyes and increased electron–hole separation at the interface between two semiconductors by establishing an inner electric field.

 Please wait while we load your content...
Please wait while we load your content...