TiO2/Bi2S3 core–shell nanowire arrays for photoelectrochemical hydrogen generation†
Abstract
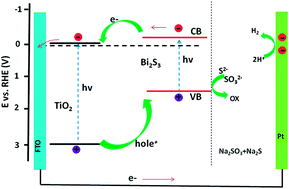
A facile and effective procedure for the synthesis of quasi-vertically aligned TiO2/Bi2S3 core–shell nanowire arrays (NWAs) is reported. The nontoxic and earth abundant Bi2S3 was assembled onto the hydrothermal pre-grown TiO2 NWAs via a successive ionic layer adsorption and reaction (SILAR) method. The morphologies, microstructures, and optical properties of the pristine TiO2 and composite TiO2/Bi2S3 with different SILAR circles were characterized in detail. For photoelectrochemical (PEC) measurements, the TiO2/Bi2S3 core–shell NWAs exhibited not only an enhanced photocurrent density (2.8 times higher than that of pristine TiO2), but also a negatively shifted onset potential from 0.067 to −0.072 V vs. RHE, as compared to the TiO2. This better PEC performance results from the broadened light absorption and the improved charge carrier separation efficiency. Our results provide a green photoelectrode for PEC hydrogen generation.

 Please wait while we load your content...
Please wait while we load your content...