A nanopoint Schottky-gate array device: surface defect application and molecular detection†
Abstract
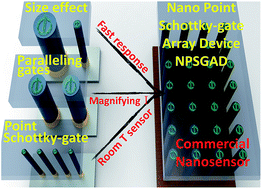
We have demonstrated a nanopoint Schottky-gate array device (NPSGAD) which can enhance the sensitivity, the signal current output level and the distinguishability by placing numerous ZnO point Schottky-gates in parallel gigantically. By using NPSGADs, we can improve the current output to the order of mA and enhance the CO sensor detection ability to room temperature detection. NPSGADs can achieve the above-mentioned properties based on several designs, e.g. point Schottky-gate can enhance detection response, parallel Schottky-gate can enhance the gas monitor levels and Schottky-gate array can reduce the total resistance for high signal current output. For UV light detection, NPSGADs can achieve a signal current of 8 mA. For room temperature detection of CO, the signal current can be enhanced above 0.25 mA. Note that these signal levels can already be used for commercial application. The gas detection signal also can be improved by increasing the operating temperature; moreover, the current variation averages of CO detection of the NPSGAD are 1.7 mA, 10.1 mA and 14.9 mA at operating temperatures of 150 °C, 350 °C and 400 °C, respectively.

 Please wait while we load your content...
Please wait while we load your content...