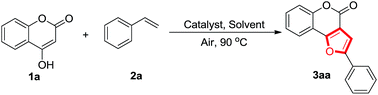
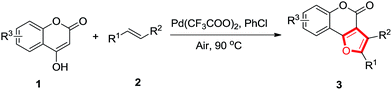Atom-economical chemoselective synthesis of furocoumarins via cascade palladium catalyzed oxidative alkoxylation of 4-oxohydrocoumarins and alkenes†
Xian-chun Tan‡
,
Hai-yuan Zhao‡,
Ying-ming Pan*,
Na Wu,
Heng-shan Wang and
Zhen-feng Chen*
Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), School of Chemistry and Pharmaceutical Sciences of Guangxi Normal University, Guilin 541004, People's Republic of China. E-mail: panym2013@hotmail.com; chenzfubc@yahoo.com; Fax: +86-773-5803930; Tel: +86-773-5846279
First published on 11th December 2014
Abstract
A novel and efficient procedure for the synthesis of furo[3,2-c]coumarins from readily available 4-oxohydrocoumarins and alkenes in the presence of a catalytic amount of Pd(CF3COO)2 has been developed. Atom-economical characteristics and mild conditions of this method are in accord with the concept of modern green chemistry.
Coumarins are important structural units in several natural products, and feature widely in biologically active compounds.1 Among them, furocoumarins are a structural motif found in numerous pharmaceutically active compounds (Fig. 1). Neo-tanshinlactone2 and Coumestrol3 exhibit high anti-tumor tissue-type as well as anti-breast cancer cell line selectivity. Pyrazolyl furocoumarin4 showed good in vitro antifungal activity.
For this reason, considerable efforts have been expended to develop the synthetic methods of furocoumarins. Sameh and Risitano groups applied respectively a tandem O-alkylation/cyclisation of 4-hydroxycoumarin and haloketones to synthesize furo[3,2-c]coumarin derivatives.5 Chen developed an efficient synthetic method via Michael–Oxa-Michael–aromatization protocol of nitroallylic acetates with 1,3-dicarbonyls mediated by base.6 Lin group employed phosphorus zwitterions with acid chloride in a one-step procedure to approach furo[3,2-c]coumarins.7 However, these methods require special materials, harsh reaction conditions, long reaction times or involving multistep synthetic operations, and all of them are not atom-economical (Scheme 1a). Therefore, an atom-economical, straightforward, convenient, and high regioselective route to synthesize furocoumarins with basic chemical materials is still highly attractive. To the best of our knowledge, the palladium-catalyzed synthesis of furo[3,2-c]coumarins directly from simple alkenes and 4-oxohydrocoumarins has not been reported so far. As a result of development on the transition-metal-catalyzed C–H functionalization of alkenes in our group,8 herein, we developed a Pd(CF3COO)2 catalyzed aerobic oxidative alkoxylation of 4-oxohydrocoumarins and alkenes in a cascade sequence to afford the desired furocoumarins in good yields (Scheme 1b).
In order to identify the optimal reaction conditions, 4-oxohydrocoumarin 1a with styrene 2a were chosen as model substrates. Initially, the reaction of 1a (0.5 mmol) and 2a (0.5 mmol) in the presence of 20 mol% Pd(CF3COO)2 in PhCl at 90 °C for 4 h gave the furo[3,2-c]coumarins 3aa in 81% yield (Table 1, entry 1). In addition, in the presence of other catalysts such as Sc(OTf)3, Y(OTf)3, Cu(OAc)2, Rh(OH)3, InCl3 and AuBr3, most of the starting material 1a was recovered (Table 1, entries 2–7). When other palladium catalysts such as Pd3(dba)2, PdCl2 and Pd(OAc)2 were used, the yield of 3aa dramatically decreased to 45−69% (Table 1, entries 8−10). Further optimization suggested that solvents also had a strong effect on this process (Table 1, entries 11−15). The reactions were obviously restrained when they were performed in DMSO, DMF and DCE (Table 1, entries 11–13). The reactions in PhCH3 and 1,4-dioxane yielded 60% and 40% of 1a, respectively (Table 1, entries 14 and 15). Hence, it was concluded that the best conditions involved 20 mol% Pd(CF3COO)2 in PhCl at 90 °C.
| Entry | Catalyst | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2a (0.5 mmol), catalyst (20 mol%), solvent (2 mL), 90 °C, 4 h.b Isolated yield of the pure product based on 1a. | |||
| 1 | Pd(CF3COO)2 | PhCl | 81 |
| 2 | Sc(OTf)3 | PhCl | 0 |
| 3 | Y(OTf)3 | PhCl | 0 |
| 4 | Cu(OAc)2 | PhCl | 0 |
| 5 | Rh(OH)3 | PhCl | 0 |
| 6 | InCl3 | PhCl | 0 |
| 7 | AuBr3 | PhCl | 0 |
| 8 | Pd3(dba)2 | PhCl | 45 |
| 9 | PdCl2 | PhCl | 30 |
| 10 | Pd(OAc)2 | PhCl | 69 |
| 11 | Pd(CF3COO)2 | DMSO | 20 |
| 12 | Pd(CF3COO)2 | DMF | 0 |
| 13 | Pd(CF3COO)2 | DCE | 25 |
| 14 | Pd(CF3COO)2 | PhCH3 | 60 |
| 15 | Pd(CF3COO)2 | 1,4-Dioxane | 40 |
With the above optimized conditions in hand, the scope of the substrates was investigated. Typical results are shown in Table 2. To our delight, catalyzed by Pd(CF3COO)2, both aryl-substituted internal alkenes and terminal alkenes produced high yields of furo[3,2-c]coumarins under the optimized conditions. Terminal alkenes with differents substituted groups such as Me, OMe, F, Cl and OCOCH3, all reacted smoothly afforded the target products in high yields (Table 2, entries 3ab–3ag). In general, the desired products could be obtained in higher yields from electron rich alkenes than that from electron poor alkenes. The position of substituent on benzene ring seemed to have little influence on the product yield (Table 2, entry 3ac). Terminal alkenes bearing polycyclic aromatic substituent, such as 2-vinylnaphthalene led to the corresponding 2-naphthalen-furo[3,2-c]coumarin 3ah in 75% yield (Table 2, entry 3ah). Additionally, the reaction of the internal alkenes 2i and 2j with 4-oxohydrocoumarin 1a also gave the desired products 3ai and 3aj in 83% and 80% yields, respectively (Table 2, entries 3ai and 3aj). Unfortunately, when the aliphatic alkene was used, the reaction failed to afford the desired product (Table 2, entry 3ak). Moreover, oxohydrocoumarin bearing substituent such as 6-methoxy-4-hydroxycoumarin and 7-methyl-4-hydroxycoumarin were found to afford the desired products in 82–84% yields (Table 2, entries 3ba-3ci).
| a Reactions conditions: 0.5 mmol of 1 and 0.5 mmol of 2 in the presence of 20 mol% of Pd(CF3COO)2, 2 mL of PhCl at 90 °C for 4 h. Isolated yields. |
|---|
 |
Surprisingly, the reaction of 4-oxohydrocoumarin 1a with different methacrylates 4 failed to afford the desired products but gave unexpected product 3al in high yields (Scheme 2).
 | ||
| Scheme 2 Synthesis of 2-methyl-furo[3,2-c]coumarin 3al from 4-hydroxycoumarin 1a and methacrylates 4. | ||
To explore the possible reaction pathway, isotope deuterium-labeled styrene 2a–d was used to react with 4-oxohydrocoumarin 1a in PhCl at 90 °C for 4 h. The substituted furo[3,2-c]coumarin 3aa–d was obtained in 79% yield. Over 96% of deuterium was incorporated in the product (Scheme 3).
On the basis of the mechanism of previous reports9 and our results, a plausible mechanism is provided in Scheme 4. α-Palladation of 1,3-ketone ester can provide A, followed by styrene coordination and Heck insertion to B. Reductive elimination to produce dihydro-furocoumarin D, which has been detected and isolated in our experiment. Finally, oxidative aromatization under air affords the desired product furocoumarins. Isolated dihydro-furocoumarin D reacted in the optimized conditions for another 2 h to afford the final furo[3,2-c]coumarin 3aa in 95% yield (Scheme 5).
In summary, we have developed an efficient synthetic method to synthesis furo[3,2-c]coumarins from readily available 4-oxohydrocoumarins and alkenes. This operationally simple method gives a rapid access to the furo[3,2-c]coumarins. Atom-economical characteristics and mild conditions of the method are in accord with the concept of modern green chemistry.
Experimental section
1H and 13C NMR spectra were measured on a Bruker Avance 500 MHz NMR spectrometer with CDCl3 as solvent and recorded in ppm relative to an internal tetramethylsilane standard. General chemicals were purchased from commercial suppliers and used without further purification.General experimental procedure for synthesis of furo[3,2-c]coumarins
The mixture of 4-hydroxycoumarin 1 (0.5 mmol), alkenes 2 (0.5 mmol) and Pd(CF3COO)2 (0.1 mmol) in PhCl (2 mL) was stirred at 90 °C for 4 h. The progress of the reaction was monitored by thin-layer chromatography. Upon completion, the mixture was then cooled and evaporated under reduced pressure. The target product 3 was purified by flash chromatography on silica gel using a mixture of ethyl acetate and petroleum ether.Acknowledgements
We thank Ministry of Education of China (IRT1225), the National Natural Science Foundation of China (21362002, 41465009 and 81260472), Guangxi Natural Science Foundation of China (2014GXNSFDA118007), State Key Laboratory Cultivation Base for the Chemistry and Molecular Engineering of Medicinal Resources, Ministry of Science and Technology of China (CMEMR2014-A02, CMEMR2014-A04, CMEMR2012-A20 and CMEMR2013-C01), Key Project of Guangxi Department of Education (2013ZD006), and Bagui Scholar Program.Notes and references
- (a) L. Santana, E. Uriarte, F. Roleira, N. Milhazes and F. Borges, Curr. Med. Chem., 2004, 11, 3239 CrossRef CAS; (b) F. Borges, F. Roleira, N. Milhazes, L. Santana and E. Uriarte, Curr. Med. Chem., 2005, 12, 887 CrossRef CAS; (c) B. M. Trost and F. Tost, J. Am. Chem. Soc., 1996, 118, 6305 CrossRef CAS.
- X. H. Wang, K. F. Bastow, C. M. Sun, Y. L. Lin, H. J. Yu, M. J. Don, T. S. Wu, S. Nakamura and K. H. Lee, J. Med. Chem., 2004, 47, 5816 CrossRef CAS PubMed.
- G. A. Kraus and N. Zhang, J. Org. Chem., 2000, 65, 5644 CrossRef CAS PubMed.
- S. Bondock, W. Khalifa and A. A. Fadda, Eur. J. Med. Chem., 2011, 46, 2555 CrossRef CAS PubMed.
- (a) A. Sehemi, G. Abdullah, E. Gogary and R. Sameh, Chin. J. Chem., 2012, 30, 316 CrossRef; (b) F. Risitano, G. Grassi, F. Foti and C. Bilardo, Tetrahedron Lett., 2001, 42, 3503 CrossRef CAS.
- W. Y. Huang, Y. C. Chen and K. Chen, Chem.–Asian J., 2012, 7, 688 CrossRef CAS PubMed.
- C. Lee, Y. Jang, Z. Wu and W. Lin, Org. Lett., 2012, 14, 1906 CrossRef CAS PubMed.
- (a) P. Liu, J. L. Liu, H.-S. Wang, Y.-M. Pan, H. Liang and Z. F. Chen, Chem. Commun., 2014, 50, 4795 RSC; (b) P. Liu, H. S. Wang, Y. M. Pan, W. L. Dai, H. Liang and Z. F. Chen, Chem. Commun., 2013, 49, 5295 RSC; (c) Q. Wu, P. Liu, Y. M. Pan, Y. L. Xu and H. S. Wang, RSC Adv., 2012, 2, 10167 RSC; (d) G. B. Huang, X. Wang, Y. M. Pan, H. S. Wang, G. Y. Yao and Y. Zhang, J. Org. Chem., 2013, 78, 2742 CrossRef CAS PubMed.
- U. Sharma, T. Naveen, A. Maji, S. Manna and D. Maiti, Angew. Chem., Int. Ed., 2013, 52, 12669 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: General experimental procedures, and spectral data, NMR spectra and high resolution mass spectra for all compounds. See DOI: 10.1039/c4ra15732j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |