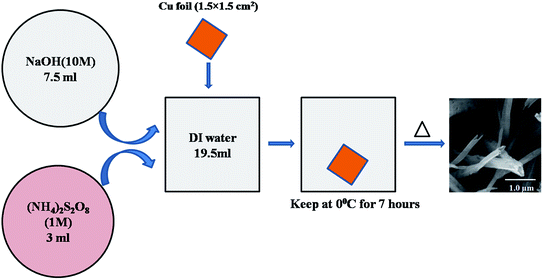
Carbon nanotube based 3-dimensional hierarchical field emitter structure
Gaurav Mittala,
Mamta Khanejab,
Krishna Sainia and
Indranil Lahiri*ac
aCentre of Nanotechnology, Indian Institute of Technology Roorkee, Roorkee-247667, India. E-mail: indrafmt@iitr.ac.in; Fax: +91-1332-285243; Tel: +91-1332-285261
bSolid State Physics Laboratory, DRDO, New Delhi-110054, India
cNanomaterials and Applications Lab., Department of Metallurgical and Materials Engineering, Indian Institute of Technology Roorkee, Roorkee-247667, India
First published on 10th February 2015
Abstract
Carbon nanotubes (CNT) are known to offer exciting electronic, electrical, mechanical and chemical properties and have found their way in a variety of applications. Field emitter is one such application in which the use of CNTs is widely appreciated. High current density field emitters with good stability have been the focus of many researchers for more than a decade. In the present study, a 3-dimensional (3D) hierarchical structure of field emitter has been demonstrated. Copper oxide (CuO) nanotubes and nanorods were synthesized directly on a copper foil substrate using a simple, easy to scale-up chemical process and CNTs were grown on these CuO nanostructures. A comparative study of the field emission behaviour of these emitters, along with its corresponding 2-dimensional (2D) structure, i.e. CNTs grown on copper foil, was performed. The basic idea behind developing the 3D architecture was to enhance the surface area available for CNT growth so that a higher emission current could be achieved without increasing the foot-print of the field emitter device. Emission current density was measured to be 0.87 mA cm−2, 3.11 mA cm−2 and 0.63 mA cm−2 for CNTs on copper foils, CNTs on CuO nanotubes and CNTs on CuO nanorods, respectively.
1. Introduction
Field emitters have a wide range of applications in field emission displays,1 microwave amplifiers,2 X-ray sources,3 electron microscopes4 and satellite propulsion systems (hall thrusters).5 High current density field emitters with good stability are appearing as a necessity for most of these applications. One dimensional nanostructures, such as nanorods, nanowires, nanotubes and nanofibers, have been extensively explored for field emission applications owing to their unique properties, including a small radius of curvature at the tip and a high aspect ratio. Among these one dimensional nanostructures, carbon nanotubes (CNTs) were investigated most extensively. They offer almost all the structural features and properties required for a good field emitter such as high aspect ratio, high conductivity, high mechanical strength, thermal stability and chemical inertness.6–10 Apart from CNTs, different nanostructures of metals like silver (Ag) nanorods,11 gold (Au) nanowires,12 copper (Cu) nanorods13 and nanowires/nanotubes of metallic oxides like ZnO,14 TiO2 (ref. 15) and CuO16,17 have also demonstrated promising field emission characteristics.High emission current density has been one of the main focus areas in the development of next-generation emitters. The implementation of 3-D architecture may be a way out in this situation, where conventional 2-dimensional architecture (with CNTs grown on a flat surface) fails to generate such high current density. Increasing the spatial density of CNTs could be an option to increase the current density. However, excessive and continuous increase in emitter density leads to a screening effect, which decreases the field emission efficiency. The main purpose of using 3-dimensional architecture is to enhance the effective surface area available for CNT growth (without affecting the foot-print area of the device) so that the emitter density can be enhanced and the screening effect could also be avoided. Fig. 1 shows a schematic of the process of creating 3-D architecture and thus increasing the surface area for the growth of CNTs. While in the 2-D architecture only the topmost surface is available for CNT growth, in the 3-D architecture, the surface area is enhanced due to the presence of some structural features on the top surface. In recent years, different varieties of 3-D emitter structures have been reported by many research groups.18–21
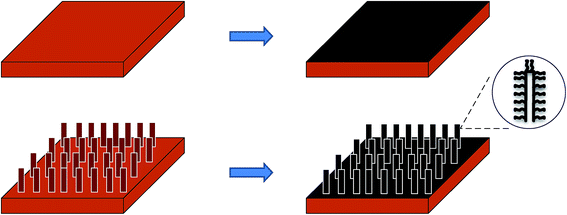 | ||
| Fig. 1 Schematic showing comparison of surface area available for CNT growth in 2-D (top) and 3-D (bottom) architecture. | ||
In the present study, a 3-dimensional hierarchical structure of field emitter was produced to obtain a high current density. In these field emitters, a CuO nanostructure–CNT hierarchical field emission array on copper foil was used. Copper was chosen as the substrate for these field emitter devices because of its high electrical conductivity (among metallic conductors, Cu (5.800 × 107 S m−1) is second to only Ag (6.287 × 107 S m−1) in electrical conductivity7) and its easy availability. CNT emitters on a copper substrate have been known to offer much better field emission properties, as compared to other metallic or semiconducting substrates.7 In the present study, nickel was chosen to be the catalyst for CNT growth, considering its higher electrical conductivity compared to other conventional catalyst materials.22 In order to prepare the hierarchical structure, the chosen structure should have a compatibility with copper, some type of conductivity and should be processed easily. Cupric oxide (CuO) was selected for this purpose, as it is a p-type semiconductor with a band gap of 1.2 eV,23 and CuO nanostructures can easily be synthesized directly on the copper foil by a simple, easy to scale-up chemical processes. Moreover, various CuO nanostructures are known to offer good field-emission properties.16,17,24,25 Thus, the selection of materials in the present study was made with the aim of developing a field emitter device, which had better emission characteristics and could be processed by a simple and easy-to-scale-up technique.
The hierarchical field emitter structure was fabricated by a two-step process. First, CuO nanostructures were grown on a copper foil by a chemical method. Then, CNTs were synthesized over using thermal chemical vapour deposition (CVD). To the best of the authors' knowledge, this Cu–CuO nanostructure–CNT hierarchical structure was demonstrated for the first time for field emitter application. The field emission behaviour of this structure was compared with samples of CNTs grown on pure copper foil (i.e. a 2-D structure), CNTs grown on CuO nanorods (referred as CuO-NR) and CuO nanotubes (referred as CuO-NT), which are considered as 3-D structures.
2. Experimental procedure
2.1 Synthesis of CuO nanotubes
A CuO-NT structure was synthesized following a modified version of the chemical route proposed by Zhang et al.23 The solutions of sodium hydroxide (NaOH) (10 mol dm−3) and ammonium persulfate ((NH4)2S2O8) (1 mol dm−3) were prepared by dissolving NaOH (98% pure) and (NH4)2S2O8 (98% pure), respectively, in deionized (DI) water. Then, 7.5 ml of the NaOH solution was mixed with 19.5 ml DI water to prepare an alkaline solution. A piece of pure copper foil (99.9% pure) of size 15 × 15 × 0.2 mm3 was bath sonicated in 50% HCl and then washed with acetone and DI water to remove impurities. One side of the copper foil was protected with an adhesive tape. Then, it was immersed in the solution prepared above. Finally, 3 ml of (NH4)2S2O8 solution was added to this reaction chamber. The entire reaction chamber was kept in a beaker containing ice and placed in a refrigerator to control the kinetics of CuO-NT formation. After 7 hours, the copper foil was taken out of the solution, washed repeatedly with DI water and dried in air.The dried samples were then heat treated in a N2 atmosphere. The samples were kept in an alumina boat and placed in the centre of a quartz tube furnace. N2 gas was purged over the samples for 10 minutes to flush the tube. Samples were heated first at 60 °C for 2 hours, then at 120 °C for 4 hours and finally at 180 °C for 6 hours in N2 atmosphere. The whole process is demonstrated in Fig. 2 with the help of a schematic.
2.2 Synthesis of CuO nanorods
A CuO-NR structure was grown on a copper foil by a simple chemical method as reported by Xue et al.26 Copper foil (15 × 15 × 0.2 mm3) was bath sonicated in 50% HCl for 10 minutes and then washed with DI water to remove surface impurities. One side of the copper foil was protected with an adhesive tape. A solution was prepared by mixing 1 ml ammonia and 80 mg NaOH in 400 ml of DI water. The washed copper foil was immersed into the prepared solution and kept at room temperature. The samples were taken out after 7 days and washed repeatedly with DI water to remove surface impurities. Dried samples were then annealed at 200 °C for 2 hours in a N2 atmosphere. Fig. 3 shows a schematic of the whole synthesis process of CuO-NR growth.2.3 Synthesis of CNTs
CNTs were grown on a pure copper foil, CuO NR structure and CuO NT structure by a thermal chemical vapour deposition (CVD) method. A solution was prepared by mixing 42 mg nickel acetate (98% pure) with 10 ml of ethanol.27 Dip coating method was used to deposit the catalyst layer onto the substrate. During dip coating, the substrate was dipped into the solution for 5 minutes and then dried in air for 10 minutes. This process was repeated 3 times so that sufficient catalyst could be deposited over the substrate. Catalyst-deposited samples were placed in an alumina tray and inserted into the CVD chamber. The chamber was flushed by purging with argon gas for 10 minutes. Samples were heated to a temperature range of 700–750 °C under a flow of 500–1000 sccm of argon and, after reaching the CVD growth temperature, acetylene was passed over the samples at a rate of 500 sccm for 10 minutes. Finally, the samples were slowly cooled to room temperature under a constant flow of Ar gas. The same procedure was repeated for all the three types of samples.2.4 Characterization
The surface morphologies and phases of the as-grown CuO-NTs and CuO-NRs were characterized using scanning electron microscopy (SEM) and X-ray diffraction (XRD), respectively. All XRD patterns were analyzed with the X'Pert Highscore software. Brunauer–Emmett–Teller (BET) method, with N2 as the adsorbate, was used to quantify the surface areas of CuO-NT and CuO-NR structures. For this purpose, CuO nanotubes and nanorods were scratched out from the copper substrate. The surfaces and morphologies of as-synthesised CNTs were characterized by SEM, Raman spectroscopy (Invia Renishaw Raman spectrophotometer with Ar laser, having a wavelength of 514 nm) and TEM (FEI TECHNAI G2 and JEOL 2100 UHR-TEM) with an operating voltage of 200 kV. For TEM observation, a dispersion of CNTs (scraped from the sample surfaces) in 5 ml acetone was used, which was bath sonicated for 10 minutes before dropping 10–20 μl of it onto copper grids. Field emission tests were performed in a parallel plate diode configuration. In the field emission measurement set-up, CNT-based samples mounted on a copper plate and fixed to a stainless steel base plate, acted as a cathode. A stainless steel disk mounted on the same fixture acted as the anode. A typical anode to cathode spacing was maintained at 500 μm. The complete diode-assembly was kept inside a vacuum chamber, which was evacuated to a base pressure of ∼1 × 10−6 torr using a turbo-molecular pump backed by a mechanical pump. This level of vacuum was maintained for all field emission tests. A gamma 3 kV dc power supply was used to apply high voltage to the test device.3. Results and discussion
3.1 CuO nanotubes and CuO nanorods
As-grown CuO nanotubes and nanorods were characterised initially by X-ray diffraction (XRD) to identify the phases formed. Fig. 4(a) shows the XRD pattern of as-synthesized CuO nanotubes. Two low-intensity diffraction peaks matched well with the available data of monoclinic CuO (JCPDS 00-001-1117), having lattice constants a = 4.653 Å, b = 3.410 Å and c = 5.108 Å. Compared with this JCPDS data, peaks at 35.74 and 38.96 could be attributed to (−1 1 1) and (1 1 1) planes of CuO, respectively. The other peaks of CuO were not visible as they have much less intensity. Another three peaks, present in Fig. 4(a) at 2θ values of 43.64°, 50.75° and 74.43°, were due to the copper substrate.Fig. 4(b) shows the XRD pattern of CuO nanorods characterized after annealing. The peaks at 32.74°, 35.74°, 38.83°, 49.04°, 53.80°, 61.67°, 67.95° and 68.16° corresponding to (−1 1 0), (0 0 2), (1 1 1), (−2 0 2), (0 2 0), (−1 1 3), (1 1 3) and (−2 2 0) matched closely with the synthetic tenorite (another variety of monoclinic CuO) structure (JCPDS 00-045-0937), with lattice constants a = 4.6853 Å, b = 3.4257 Å and c = 5.1303 Å. All the remaining 2θ peaks (43.48, 50.59 and 74.29) belong to the copper substrate.
The morphology and structure of CuO-NTs and CuO-NRs, as characterised by FE-SEM, are shown in Fig. 5. Fig. 5(a) shows images of the CuO nanotubes, which were uniformly grown on the surface of copper foil. CuO nanotubes were found to be vertically erect on the substrate. The mean diameter of the nanotubes was measured to be 315 ± 83 nm and lengths of several micrometers. The higher magnification SEM image (inset of Fig. 5(a)) clearly shows the nanotube formation. The lower reaction temperature (0 °C), used for the synthesis of CuO-NTs, decreases the reaction rate and the surface of the copper foil is uniformly covered with the characteristic blue film of Cu(OH)2.23 On annealing, Cu(OH)2 is converted to CuO. Fig. 5(b) presents a low magnification SEM image of the CuO nanorods. In contrast to CuO-NTs, the nanorods were observed to be randomly oriented and were also not uniformly distributed over the whole substrate.
 | ||
| Fig. 5 FE-SEM images of the samples. (a) CuO nanotubes (inset is the higher magnification image of the same sample showing nanotube structure formation) and (b) CuO nanorods. | ||
The surface area of the as-synthesized nanostructures was calculated using Brunauer–Emmett–Teller (BET) method. CuO NTs and NRs were grown on same sized copper foil samples, i.e. 1.5 × 1.5 cm2 and scratched out of the substrate surface to measure the surface area. The calculated surface area was measured to be 59.465 m2 g−1 and 37.185 m2 g−1 for CuO NTs and CuO NRs, respectively (Fig. 6), as compared to a very low value of 5.61 cm2 g−1 for 2-D copper foil.
3.2 Synthesis of carbon nanotubes on different substrates
Fig. 7 shows the FE-SEM images of the as-synthesized CNTs on different samples. Fig. 7(a) shows randomly oriented multiwall carbon nanotubes (MWCNT) grown on pure copper foil. The mean diameter of these MWCNTs was measured to be 48 ± 6 nm, as shown in the diameter histogram plot (Fig. 7(d)). CNTs were also grown on CuO NT and NR structures using the same CVD parameters as used for pure copper foil. FE-SEM images of these are shown in Fig. 7(b) and (c). The diameter of these MWCNTs was measured to be 89 ± 23 nm and 69 ± 7 nm for CuO NTs and CuO NRs, respectively, as represented by the histogram plots in Fig. 7(e) and (f).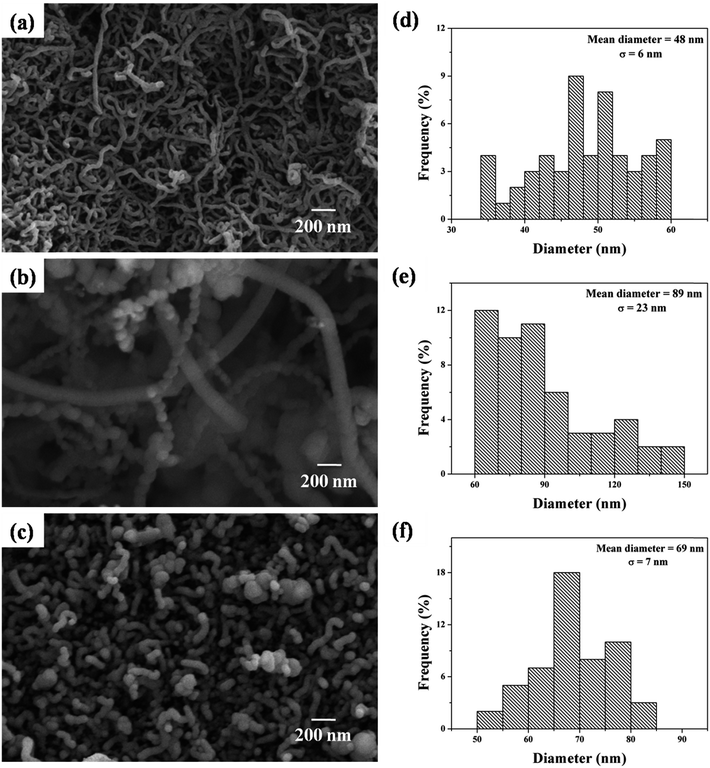 | ||
| Fig. 7 FE-SEM images of CNTs grown on (a) copper foil, (b) CuO NTs and (c) CuO NRs; the diameter distribution histograms of CNTs grown on (d) copper foil, (e) CuO NTs and (f) CuO NRs. | ||
To better understand the nature of the CNT structures, TEM and Raman spectroscopy were performed. Fig. 8(a) shows the Raman spectra of carbon nanotubes grown on copper foil. First-order Raman spectra of all graphite-like materials, including MWCNTs, show peaks around 1580 cm−1 (G-band, from graphite like sp2 bonds) and 1350 cm−1 (D-band, from diamond like sp3 bonds).28 In the present study, peaks were shifted to 1590.89 cm−1 and 1346.71 cm−1, indicating a significant amount of crystallinity and defect present in the material. The structural purity of the CNT materials is most popularly characterized by the ID/IG peak ratio. The high ratio (ID/IG = 1.113) in the present study indicates high defect density in the MWCNTs' structure. Raman spectra for the sample did not show any peak at the RBM band, depicting an absence of SWCNTs. The TEM image shown in Fig. 8(b), throws direct light on the presence of a clear-cut tubular structure in these MWCNTs.
 | ||
| Fig. 8 (a) Raman spectra of the MWCNTs on copper foil with characteristic peaks at 1346.71 cm−1 and 1590.89 cm−1, (b) a representative TEM image of the as-synthesized CNT. | ||
3.3 Field emission characteristics
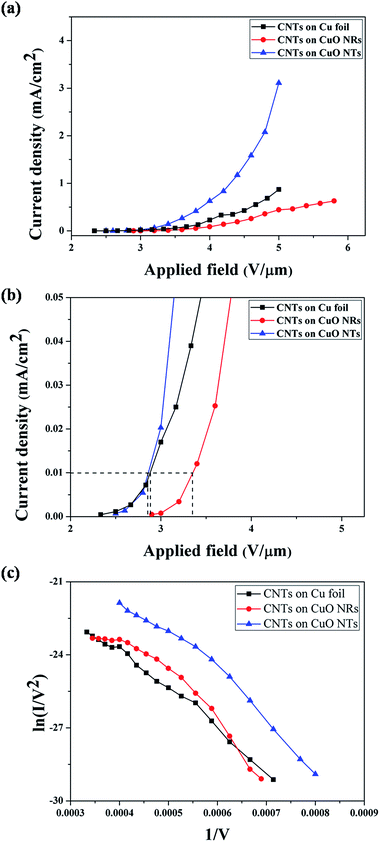
All field emission tests were performed under DC bias. The field emission characteristics of these MWCNTs based samples are presented in Fig. 9. Fig. 9(a) shows current density vs. applied field (J–E), while Fig. 9(b) presents an enlarged view of this curve to identify the turn-on fields of these emitters. Fowler–Nordheim (F–N) plots are presented in Fig. 9(c).The turn-on field (defined as the electric field required to achieve emission current density of 10 μA cm−2) was found to be 2.88 V μm−1, 2.86 V μm−1 and 3.36 V μm−1 for CNTs grown on pure copper foil, CuO nanotubes and CuO nanorods, respectively. In this respect, the CNTs–CuO NR–Cu emitter structure was found to be much worse than the other two structures, a behaviour which can be related to the highly inhomogeneous growth of CuO NRs. However, the nearly straight line nature of all the F–N plots, shown in Fig. 9(c), indicates that the emission mechanism, for all the emitters, was essentially electron tunnelling. The field enhancement factor (β) was calculated using Fowler–Nordheim (F–N) equation, i.e., I = (aAβ2E2/ϕ)exp(−bϕ3/2/βE).27 From the F–N plot, the field enhancement factor has been calculated as 4841, 4408 and 4540 for CNTs grown on pure copper foil, CuO nanotubes and CuO nanorods, respectively. This observation can be reconfirmed by the calculations shown by Xu et al.,36 which shows decrease in field enhancement factor as the radius of MWCNT increases.
Emission current density was recorded to be 0.87 mA cm−2, 3.11 mA cm−2 and 0.63 mA cm−2 at applied electric fields of 5 V μm−1, 5 V μm−1 and 5.8 V μm−1 for CNTs on copper foil, CuO nanotubes and CuO nanorods (0.44 mA cm−2 at 5 V μm−1), respectively, as shown in Fig. 9(a). It was also observed that the emission current density from CNTs grown on CuO NTs was much higher than other samples, which may be related to the large surface area of CuO nanotubes (as confirmed by BET, Fig. 6). The larger surface area of CuO-NTs prompted the growth of more CNTs on them, which in turn led to higher emission current density. On the other hand, the current density from CNTs grown on CuO NRs was very low, even lower than the conventional 2-D emitter structure. This observation may be related to the inhomogeneity of the CuO NRs' structure, which led to the non-uniform growth of CNTs and hence, a poor field emission response.
The variation in emission current from CNT–CuO NT–Cu and CNT–CuO NR–Cu samples can also be correlated with the diameter distribution of grown CNTs. Although the field enhancement factor decreases as the radius of the CNT increases, as calculated by Xu et al.;36 however, in the case of large radii CNTs, the emission current increases as the radius of the MWCNTs increases, due to increment in the emitting area.37 Hence, the CNT–CuO NT–Cu emitters, which have the largest radii, emit the maximum current among the three structures studied. Even if the MWCNTs were of the same size, the CNT–CuO NT–Cu emitter is anticipated to demonstrate better emission properties, simply owing to its higher surface area and greater number of CNT emitters (Table 1).
| Sample | Surface area (m2 g−1) | CNT diameter (nm) | Turn-on field (V μm−1) | Emission current density (mA cm−2) | Field enhancement factor (β) |
|---|---|---|---|---|---|
| CNTs on Cu foil | 0.0056 | 48 ± 6 | 2.88 | 0.87 | 4841 |
| CNTs on CuO nanotubes | 59.465 | 89 ± 23 | 2.86 | 3.11 | 4408 |
| CNTs on CuO nanorods | 37.185 | 69 ± 7 | 3.36 | 0.63 | 4540 |
Results obtained in the present study show the best emission properties from the CNT–CuO NT–Cu emitter structure. The turn-on field (2.86 V μm−1) obtained from this sample was better than many other emitter structures, including another variety of hierarchical structure of ZnO nanoneedles on ZnO nanofiber (Table 2). However, the turn-on field was found to be more than most of the nanostructured carbon material based emitters. This observation can be related to the presence of CuO in our emitter structure, which is known to be a p-type semiconductor, offering higher resistance than the nanostructured carbon materials (CNTs or graphene). On the other hand, the emission current density, obtained from our sample, was found to be much higher than most of the emitters, as shown in Table 2. Herein, it can be recalled that the aim of the present study was to get high emission current density from a hierarchical emitter structure, which appears to be fulfilled. However, there is ample opportunity to further improve the emission current density and efforts are underway to address this issue. The present study clearly proves the effectiveness of a hierarchical structure over conventional 2-dimensional emitters. Furthermore, the simplicity of the process used to prepare this hierarchical emitter structure makes it a promising emitter material for industrial application. Moreover, the processing technique involves the direct synthesis of CuO-NTs on copper foil and direct growth of CNTs onto it. All these direct growth processes ensure that the emitters are bonded well with the substrates, which is an important factor for the lifetime of any device.28
| Sr. no. | Material | Turn on field | Emission current density | Reference |
|---|---|---|---|---|
| 1 | Ar plasma treated FLG sheet on Si | 2.23 V μm−1 | 1.3 mA cm−2 | 29 |
| 2 | N-doped graphene on Si substrate | 0.6 V μm−1 | 10 μA cm−2 | 30 |
| 3 | MWCNT on Cu substrate | 0.6 V μm−1 | Total emission current = 1.5 mA | 31 |
| 4 | B-doped CNT (7 × 7 mm2) on Si substrate | 3 kV | Current density is 4 A cm−2 for total emission current = 1 μA | 32 |
| 5 | SWCNT grown on textured Si substrate | 1.3–2.4 V μm−1 at 1 μA cm−2 | 0.1–1.2 mA cm−2 | 33 |
| 6 | BN nanotube deposited on as grown graphite nanofibre | 0.72 V μm−1 | Total emission current = 89 μA | 34 |
| 7 | BN nanotubes on Si substrate | 8.3 V μm−1 | — | 35 |
| 8 | ZnO nanoneedles on ZnO nanofibre | 4.8 V μm−1 | — | 21 |
| 9 | 3-D metal–graphene-nanotube | 0.26 V μm−1 | 12.67 mA cm−2 | 19 |
4. Conclusion
In the present study, a 3-dimensional hierarchical field emitter structure has been fabricated successfully, in which CNTs have been synthesised on CuO nanotubes and nanorods structures, which in turn were synthesized directly onto copper foil. A comparative study between CNTs on copper foil (i.e. 2-D structure) and CNTs on CuO NTs and NRs (i.e. 3-D structure) showed a great promise of 3-D structures. The hierarchical structure having CNT–CuO NT–Cu registered more than 250% increase in the emission current density as compared to CNTs directly grown on copper foil. The enhancement in emission current density of the 3-D hierarchical structure was related to the higher surface area of the CuO-NT structure and its uniform distribution over copper foils.Acknowledgements
G.M. would like to thank Dr Anil Kumar, Head, Department of Chemistry, IITR for allowing to use the Raman spectrometer and Dr P. Jeevanandam, Department of Chemistry, IITR for allowing to use BET. G.M. would also like to thank the Head, Institute Instrumentation Centre, IITR for allowing access to various analytical facilities. M.K. wishes to thank the nanotechnology group of SSPL for providing support in field emission tests. This study was funded by IL's IIT Roorkee faculty initiation grant (Grant no. FIG/100612).References
- W. B. Choi, D. S. Chung, J. H. Kang, H. Y. Kim, Y. W. Jin, I. T. Han, Y. H. Lee, J. E. Jung, N. S. Lee, G. S. Park and J. M. Kim, Appl. Phys. Lett., 1999, 75, 3129 CrossRef CAS PubMed.
- K. B. K. Teo, E. Minoux, L. Hudanski, F. Peauger, J. P. Schnell, L. Gangloff, P. Legagneux, D. Dieumegard, G. A. J. Amaratunga and W. I. Milne, Nature, 2005, 437, 968 CrossRef CAS PubMed.
- J. Zhang, G. Yang, Y. Cheng, B. Gao, Q. Qiu, Y. Z. Lee, J. P. Lu and O. Zhou, Appl. Phys. Lett., 2005, 86, 184104 CrossRef PubMed.
- N. de Jonge, Y. Lamy, K. Schoots and T. H. Oosterkamp, Nature, 2002, 420, 393 CrossRef CAS PubMed.
- F. G. Rüdenauer, Surf. Interface Anal., 2007, 39, 116 CrossRef.
- W. A. de Heer, A. Châtelain and D. Ugarte, Science, 1995, 270, 1179 CAS.
- I. Lahiri, R. Seelaboyina, J. Y. Hwang, R. Banerjee and W. Choi, Carbon, 2010, 48, 1531 CrossRef CAS PubMed.
- P. G. Collins and A. Zettl, Appl. Phys. Lett., 1996, 69, 1969 CrossRef PubMed.
- Q. H. Wang, A. A. Setlur, J. M. Lauerhaas, J. Y. Dai, E. W. Seelig and R. P. H. Chang, Appl. Phys. Lett., 1998, 72, 2912 CrossRef CAS PubMed.
- H. Dai, Acc. Chem. Res., 2002, 35, 1035 CrossRef CAS PubMed.
- C. Indrani and A. Pushan, Nanotechnology, 2012, 23, 015704 CrossRef PubMed.
- A. Dangwal, C. S. Pandey, G. Müller, S. Karim, T. W. Cornelius and C. Trautmann, Appl. Phys. Lett., 2008, 92, 063115 CrossRef PubMed.
- F. Maurer, A. Dangwal, D. Lysenkov, G. Müller, M. Eugenia Toimil-Molares, C. Trautmann, J. Brötz and H. Fuess, Nucl. Instrum. Methods Phys. Res., 2006, 245, 337 CrossRef CAS PubMed.
- C. J. Lee, T. J. Lee, S. C. Lyu, Y. Zhang, H. Ruh and H. J. Lee, Appl. Phys. Lett., 2002, 81, 3648 CrossRef CAS PubMed.
- Y. Alivov, M. Klopfer and S. Molloi, Appl. Phys. Lett., 2011, 99, 063104 CrossRef PubMed.
- S. C. Yeon, W. Y. Sung, W. J. Kim, S. M. Lee, H. Y. Lee and Y. H. Kim, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.–Process., Meas., Phenom., 2006, 24, 940 CrossRef CAS.
- R. Z. Zhan, J. Chen, S. Z. Deng and N. S. Xu, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.–Process., Meas., Phenom., 2010, 28, 558 CrossRef CAS.
- I. Lahiri, J. Wong, Z. Zhou and W. Choi, Appl. Phys. Lett., 2012, 101, 063110 CrossRef PubMed.
- Z. Yan, L. Ma, Y. Zhu, I. Lahiri, M. G. Hahm, Z. Liu, S. Yang, C. Xiang, W. Lu, Z. Peng, Z. Sun, C. Kittrell, J. Lou, W. Choi, P. M. Ajayan and J. M. Tour, ACS Nano, 2012, 7, 58 CrossRef PubMed.
- N. Liu, G. Fang, W. Zeng, H. Long and X. Zhao, J. Phys. Chem. C, 2011, 115, 14377 CAS.
- Y. Liu, Y. Xie, J. Chen, J. Liu, C. Gao, C. Yun, B. Lu and E. Xie, J. Am. Ceram. Soc., 2011, 94, 4387 CrossRef CAS PubMed.
- I. Lahiri and W. Choi, Acta Mater., 2011, 59, 5411 CrossRef CAS PubMed.
- W. Zhang, S. Ding, Z. Yang, A. Liu, Y. Qian, S. Tang and S. Yang, J. Cryst. Growth, 2006, 291, 479 CrossRef CAS PubMed.
- C. T. Hsieh, J. M. Chen, H. H. Lin and H. C. Shih, Appl. Phys. Lett., 2003, 83, 3383 CrossRef CAS PubMed.
- J. Chen, S. Z. Deng, N. S. Xu, W. Zhang, X. Wen and S. Yang, Appl. Phys. Lett., 2003, 83, 746 CrossRef CAS PubMed.
- X. Xue, P. Deng, S. Yuan, Y. Nie, B. He, L. Xing and Y. Zhang, Energy Environ. Sci., 2013, 6, 2615 CAS.
- (a) U. Husnu Emrah and C. Manish, Nanotechnology, 2005, 16, 2153 CrossRef PubMed; (b) I. Lahiri, V. P. Verma and W. Choi, Carbon, 2011, 49, 1614 CrossRef CAS PubMed.
- (a) E. F. Antunes, A. O. Lobo, E. J. Corat, V. J. Trava-Airoldi, A. A. Martin and C. Verissimo, Carbon, 2006, 44, 2202 CrossRef CAS PubMed; (b) I. Lahiri, D. Lahiri, S. Jin, A. Agarwal and W. Choi, ACS Nano, 2011, 5, 780 CrossRef CAS PubMed.
- J. L. Qi, X. Wang, W. T. Zheng, H. W. Tian, C. Q. Hu and Y. S. Peng, J. Phys. D: Appl. Phys., 2010, 43, 055302 CrossRef.
- U. A. Palnitkar, R. V. Kashid, M. A. More, D. S. Joag, L. S. Panchakarla and C. N. R. Rao, Appl. Phys. Lett., 2010, 97, 063102 CrossRef PubMed.
- I. Lahiri, R. Seelaboyina, J. Y. Hwang, R. Banerjee and W. Choi, Carbon, 2010, 48, 1531 CrossRef CAS PubMed.
- R. B. Sharma, D. J. Late, D. S. Joag, A. Govindraj and C. N. R. Rao, Chem. Phys. Lett., 2006, 428, 102 CrossRef CAS PubMed.
- Y. Shiratori, K. Furuichi, S. Noda, H. Sugime, Y. Tsuji, Z. Zhang, S. Maruyama and Y. Yamaguchi, Jpn. J. Appl. Phys., 2008, 47, 4780 CrossRef CAS.
- C. Kimura, T. Yamamota, S. Funakawa, M. Hirakawa, H. Murakami and T. Sugino, J. Vac. Sci. Technol., B: Nanotechnol. Microelectron.: Mater., Process., Meas., Phenom., 2012, 21, 2212 CrossRef.
- T. Sugino, C. Kimura and T. Yamamota, Appl. Phys. Lett., 2002, 80, 3602 CrossRef CAS PubMed.
- Z. Xu, X. D. Bai and E. G. Wang, Appl. Phys. Lett., 2006, 88, 133107 CrossRef PubMed.
- A. Mayer, N. M. Miskovsky and P. H. Cutler, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 65, 155420 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |