Hydrophilic modification of polyvinyl chloride hollow fiber membranes by silica with a weak in situ sol–gel method
Abstract
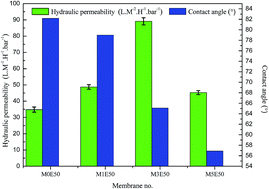
A weak in situ sol–gel method is proposed for the hydrophilic modification of polyvinyl chloride (PVC) hollow fiber membranes by silica, which is generated by the soft hydrolysis of tetraethoxysilane (TEOS) in a deionized water bath. The silica is uniformly distributed on the membrane surface. The sponge-like structure of the modified PVC membranes becomes thicker with the addition of TEOS. The surface hydrophilicity of the membranes gradually increases due to the introduction of silica. The hydraulic permeability increases from 34.8 L M−2 H−1 bar−1 to 89.1 L M−2 H−1 bar−1, and then decreases to 45.3 L M−2 H−1 bar−1 for the membranes of M0(1,3,5)E50 with the addition of TEOS from 0 to 5 wt% in dope content when 50 wt% ethanol aqueous solution is used as the bore liquid. A similar tendency is found for the membranes M0(1,3,5)D95 with 95 wt% DMAc aqueous solution as the bore liquid. The anti-fouling experiments illustrate that the membranes with the addition of TEOS show higher anti-fouling ability. Moreover, the mechanical properties of PVC membranes are also enhanced with the introduction of silica. This work demonstrates that PVC inorganic–organic composite hollow fiber membranes are prepared by a weak in situ sol–gel method, which avoids the use of corrosive substances during membrane preparation.

 Please wait while we load your content...
Please wait while we load your content...