Structure resembling effect of clay surface on photochemical properties of meso-phenyl or pyridyl-substituted monocationic antimony(v) porphyrin derivatives†
Abstract
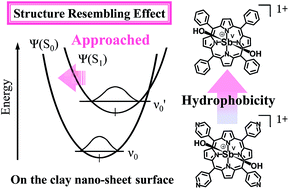
Four types of meso-phenyl or pyridyl-substituted monocationic antimony(V) porphyrin derivatives (SbVPors)—5,10,15,20-tetraphenyl; 5,10,15-triphenyl-20-mono(4-pyridyl); 5,15-diphenyl-10,20-di(4-pyridyl); and 5,10,15,20-tetra(4-pyridyl)porphyrinato dihydroxo antimony(V) chloride—with different hydrophobicities were synthesised, and their photochemical properties on anionic clay were investigated. The absorption and fluorescence behaviour of the SbVPors were strongly affected by complex formation with clay. Interestingly, the absorption transition probabilities and fluorescence quantum yields of the SbVPors prominently increased on the clay surface. The more hydrophobic SbVPor showed greater absorption transition probability increase and fluorescence quantum yield enhancement. These unique effects of the highly flat clay surface on the photochemical behaviour of SbVPor were discussed mainly from the viewpoint of transition probability, by using the potential energy curves of SbVPor with and without clay. For the more hydrophobic SbVPor, the molecular structure of the ground and excited states on the clay surface tended to become similar because of the strong hydrophobic interaction between porphyrin and the clay surface, i.e. the ‘structure resembling effect’. This effect induces a change in the transition probabilities.

 Please wait while we load your content...
Please wait while we load your content...